Dr. O’Neal is collecting data on the success of Project 4. After you have isolated your products and finished your analysis, please report your yields and any comments in this data table.
Part A: Chalcone Synthesis
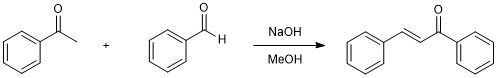
Generic synthesis of chalcones.
Acetophenone (5 mmol), benzaldehyde (5 mmol), and methanol (15 mL) were combined in a 250 mL Erlenmeyer flask equipped with a magnetic stir bar. Aqueous sodium hydroxide (30% m/v, 2.0 mL) was added dropwise. The reaction mixture was stirred at room temperature for 20-30 minutes at which point a solid precipitated. The flask was chilled in an ice-water bath, then ice-cold water (~50 mL) was added. The precipitate was collected by vacuum filtration and washed well with cold water followed by cold methanol.
NOTES
1. If no solid precipitates after stirring the reaction mixture at room temperature for 30 minutes, heat the mixture to a gentle reflux for 5-10 minutes. Solid should then precipitate upon cooling. If not, try adding a little water to induce precipitation.
2. Remember that you need to make a convincing argument for the structure of your chalcone. You may decide which characterization data to obtain in order to support your argument. Most chalcones are known compounds with characterization data already reported in the literature.
Part B: Isoxazole Synthesis
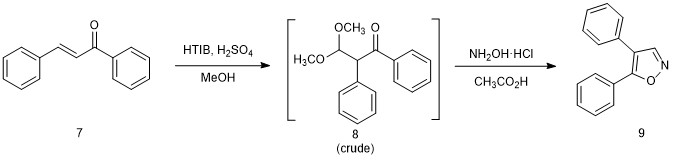
Generic synthesis of isoxazoles.
Chalcone (1 mmol) was suspended in methanol (20 mL). Hydroxy(tosyloxy)iodobenzene (HTIB, 3 mmol) and 50% sulfuric acid in methanol (0.28 mL) were added, and the mixture was refluxed until the chalcone was no longer evident by TLC (30 min, 8:2 hexane:ethyl acetate). The solvent was removed by rotary evaporation, and the crude mixture was reconstituted in glacial acetic acid (10 mL). Hydroxylamine hydrochloride (3 mmol) was added, and the mixture was refluxed until isoxazole formation was complete by TLC (45 min, 8:2 hexane:ethyl acetate). The flask was cooled to room temperature. (If necessary, this may be a stopping point for the week. See Note 2.) The reaction mixture was poured into ethyl acetate (30 mL). The mixture was washed sequentially with water (2 × 30 mL), saturate sodium bicarbonate (30 mL) and brine, dried over sodium sulfate, gravity filtered, and concentrated by rotary evaporation. The resulting oil was dissolved in methanol (2-3 mL) and poured into a 100 mL beaker. Upon standing, the product solidified. The mixture was chilled in an ice-water bath and the solids were collected by vacuum filtration, washing well with hexane. Recrystallization from hexane gave purified isoxazole.
NOTES & HINTS
1. Scale your reaction such that you use all of the chalcone you synthesized in Part A.
2. If you run out of lab time, the isoxazole in acetic acid reaction mixture can be saved for workup the following week. Pour the mixture into an Erlenmeyer flask, cover it with parafilm, add a label, and leave it in the fume hood at the front of the lab.
3. The final oil obtained after rotary evaporation is a mixture of product and iodobenzene generated during the oxidative rearrangement. The iodobenzene complicates the isolation of the isoxazole and solidification may take a long time. If necessary, leave the open beaker in the back of your hood for the week to allow the product to crystallize. If there is still no solid when you return the following week, you may need to isolate your product by column chromatography.
