Your laboratory notebook is the original, authentic, permanent, legal record of your laboratory experience. The guiding principle for composing a laboratory notebook is that the document should allow another individual with your level of experience to replicate your experiment or investigation. In this course, you must use an electronic lab notebook in the form of a MS Word document that is shared with your instructor on Box. This document is different from a notebook that you might use in a research setting because it is designed using a workbook-style format that prompts you to include specific entries and answer key questions in your data analysis.
For any laboratory activity, notebook entries are made in three distinct phases. Pre-lab entries describe the objective and plan for an experiment, and they should simplify the task of recording data while the experiment is in progress. In-lab entries should be a running account of the experiment as it is performed. Post-lab entries are written after the experiment is complete and are meant to interpret and summarize the collected data.
General Guidelines
1. Entries should be made directly in the notebook as the experiment is performed. Information should never be recorded on separate pieces of paper and then transcribed into the notebook.
2. Recognize that the notebook is a working document. It is not a piece of finished artwork. All laboratory notebooks contain mistakes and notes of explanation or clarification.
3. Mistakes should be corrected but never removed. A single line should be used to strike through any error. Changes in primary data (e.g., the mass of a reactant) are usually initialed and accompanied by a note of explanation.
General Guidelines for Pre-lab Entries
1. The title should convey the primary task performed during the experiment.
2. Each project should include the date on which the experiment was performed.
3. Lab Partner. If you are working in a group, the name(s) of your lab partner(s) should be recorded.
4. Purpose statement. Briefly summarize the scientific objective(s) of the experiment.
5. Background. Appropriate background information varies widely with the type of experiment. In general, you should include any information necessary for someone with your expertise to understand and accomplish the lab. Typical background information includes a chemical equation, important data for the materials used, and any relevant calculations (i.e., theoretical yield). It might also include a description of the technique or instrument employed during the experiment or the mechanism of a reaction. Safety precautions should be clearly noted in the background section. The three most common forms of background information in this course are:
• Reagent Table: List any chemicals used in the experiment with relevant physical and safety data (e.g., molecular weights, quantities used, densities, hazards, precautions, etc.).
• Reaction Equation: If the experimental objective involves a chemical transformation, a corresponding chemical equation should be provided. The equation should illustrate the reactants, reagents, conditions and products.
• Reaction Data Table: For substances used in a chemical reaction, include a table that provides information about stoichiometric ratios. The sample laboratory notebook provides an example of a typical reagent table.
6. Procedure Outline. Outline the intended procedure in enough detail for you to follow easily when you enter the lab. You may use a list or flow chart format in this section. The procedure section in your notebook is divided into columns. You may find it helpful to record your planned procedure in one column, leaving the adjacent space free to record any procedural alterations and data gathered. It is often helpful to include pre-designed data tables to simplify the task of recording data while completing the experiment.
General Guidelines for In-lab Entries
1. Fully describe your actions during the lab. If you have a well-written procedure outline, you simply need to record any planned or unplanned deviations from the protocol as you carry out the lab.
2. As you carry out the procedure, you should record the raw data you collect directly into your notebook. This includes any measurements taken and the actual quantities of materials used, which will inevitably vary somewhat from the planned quantity in the procedure.
3. Numbers are not the only type of data you gather in the lab. It is important to record what you observe (e.g., precipitates, color changes, bubbles, heat generated, smells, etc.) during the course of the experiment.
General Guidelines for Post-lab Entries
Data Analysis. After the experiment is complete and all the data is gathered, you must examine it to determine its meaning. Raw data often must be manipulated in some way. Record your calculations or analysis of the data so that someone else could follow you reasoning and confirm your results. The Data Analysis section of your lab notebook is formatted in a workbook style to prompt you to think carefully about the relevant data gathered in each experiment.
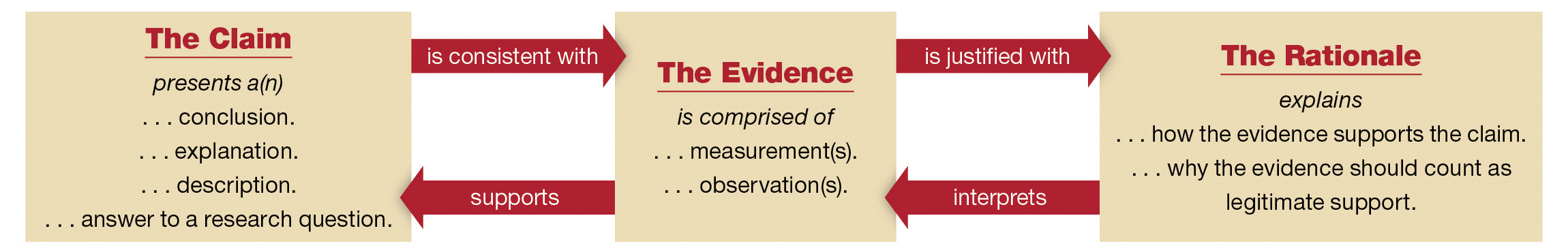
Conclusion. The conclusion section is designed to help you think broadly about the outcome of your project. This section prompts you to use the combination of data gathered to make general claims about your findings. Note that claims should be simple, direct statements that you support by citing the relevant data and briefly explaining how the data supports the claim. Remember that you will have provided a detailed analysis of each data source in the Data Analysis section. You do NOT need to repeat that analysis in the Conclusion section. Simply state in broad terms how the data support your claim. The graphic below illustrates the relationship between the three components of a strong scientific conclusion: claim, evidence, and rationale. As the figure illustrates, the claim must be consistent with the evidence. The rationale explicitly interprets the evidence so that the reader can follow the logic of how it supports the claim.
Summary and Reflections. This section of the notebook is designed to help you connect the major outcomes you observed (summarized in the Conclusion section(s)) with the overall goal of the project. The Summary is a brief paragraph that describes this connection and expresses the major findings of the project. The Reflections questions are a brief set of open-ended questions about the chemistry and skills related to a particular project. These will push you to critically analyze the experiment and make connections with the theories and concepts you encounter in lecture.
