Dr. O’Neal is collecting data on the success of Project 2. After you have isolated your products and finished your analysis, please report your yields and any comments in this data table.
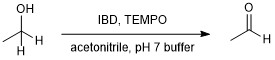
In a round bottom flask, the alcohol (3.2 mmol) was dissolved in acetonitrile (4 mL). The following reagents were then added sequentially: aqueous pH 7.0 buffer (0.768 mL), 2,2,2,6-tetramethylpiperidin-1-oxyl (TEMPO) (0.32 mmol), and iodobenzene diacetate (IBD) (3.6 mmol). The reaction was stirred at room temperature, and progress was monitored at 10 minute intervals by TLC (8:2 hexane:ethyl acetate). When the reaction was complete, 95% ethanol (16 mL) was added followed by 3.8 M sodium bisulfite solution (4 mL). The mixture was stirred vigorously in an ice-water bath. After a solid was observed to precipitate, stirring was continued for an additional 15 min. The solid was then collected by vacuum filtration and washed well with ice-cold 95% ethanol. The solid derivative was transferred to a 100 mL round bottom flask and dissolved in water (40 mL). Ethyl acetate (20 mL) was added to the flask, and the mixture was refluxed for 30 minutes. After cooling slightly, the mixture was poured into a separatory funnel, and the layers were separated. The aqueous layer was extracted with ethyl acetate (2 x 15 mL). The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and concentrated by rotary evaporation. The resulting product was characterized by IR, GC-MS, and NMR.
Reminders & Hints
1. You must complete the Project 2A Prelab Quiz before entering the lab to perform this procedure.
2. You must prepare your own 3.8 M sodium bisulfite solution. Be sure to calculate the quantity of sodium bisulfite you need to dissolve into 4 mL of water to prepare this solution. You should make the solution as soon as you set up your reaction mixture so that it is ready to use as soon as your data indicates that the reaction is complete.
3. Recall that “washing” is the same as a liquid-liquid extraction. The term is applied when the desired substance is NOT expected to migrate into the solution being added (e.g., brine). So, you add the solution (e.g. brine), mix the two layers, allow the layers to separate, and drain away the undesired solution.
4. Don’t forget that you need to determine the mass of your final product. Pre-weigh the round-bottom flask before the final rotary evaporation step to simplify this measurement!
