Make Your Own Molecule
A Real-World Research Project
Isoxazoles are five-membered heteroaromatic compounds that contain adjacent oxygen and nitrogen atoms. Many isoxazole-containing compounds are known to exhibit potent biological activities, including anticancer, antibacterial, antifungal, antiviral, anti-tuberculosis, and antiflammatory properties. Some examples of these compounds are illustrated in Figure 1.
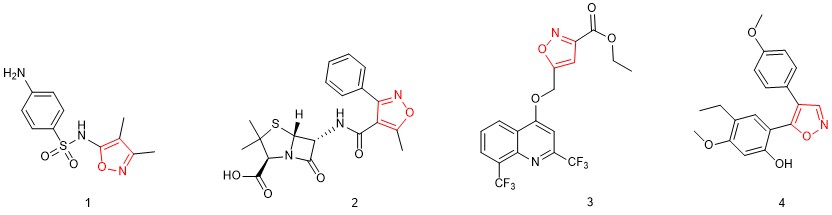
Figure 1. Some examples of biologically active isoxazoles. Sulfisoxazole (1) is an antibiotic used to treat a range of severe bacterial infections. Oxacillin (2) is a penicillin antibiotic most often used to treat resistant staphylococci infections. Compound 3 was reported as a potential prodrug to treat tuberculosis infections. KRIBB3 (4) shows significant anti-tumor activity against a variety of cancers.
KRIBB3 (4) is a relatively simple 4,5-diarylisoxazole that displays significant antitumor activity against colon, prostate, breast, and lung cancers. This compound and similar isoxazole analogs may be attractive candidates for clinical applications.
A University of Richmond researcher recently demonstrated an effective method for preparing the simplest KRIBB3 analog, 4,5-diphenylisoxazole (Figure 2). The synthesis technically involves three reaction steps, but the second two steps are combined into a “one-pot” synthesis. In a one-pot synthesis, the reactant undergoes successive chemical transformations in the same reaction vessel, thereby increasing the efficiency of the overall process.
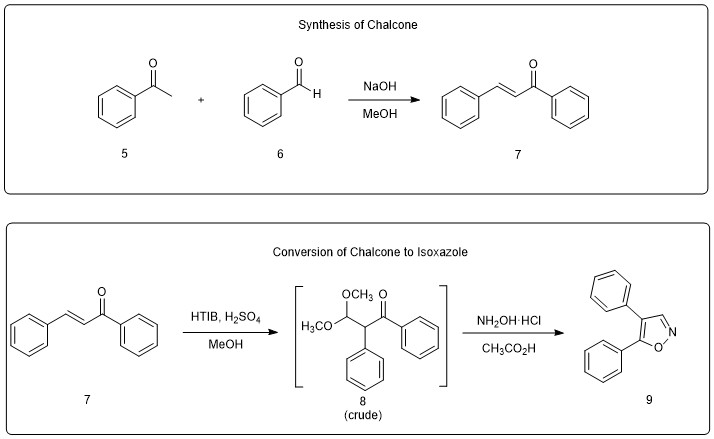
Figure 2. Synthesis of 4,5-diphenylisoxazole (9) via oxidative rearrangement and cyclization of chalcone.
The synthesis begins with the condensation of acetophenone 5 and benzaldehyde 6 to generate chalcone 7. Note that chalcone refers to the generic structural motif of compound 7. Molecules that share this core but also have substituents on the aromatic rings are all generically referred to as chalcones.
Since isoxazoles are in a higher oxidation state than chalcones, an oxidizing agent is required at some point during any conversion of a chalcone to an isoxazole. Hydroxy(tosyloxy)iodobenzene (a.k.a. HTIB) induces a simultaneous oxidation and rearrangement of chalcones in which the two aromatic rings end up on adjacent carbon atoms. Under the reaction conditions employed for Project 4, the chalcone rearranges to a dimethylacetal of type 8. After removal of the methanol solvent, crude acetal 8 undergoes condensation with hydroxylamine hydrochloride in acetic acid to produce the isoxazole (9).
When organic chemists develop a new reaction method, one of the first things they investigate is the scope of the reaction. The basic questions are: If I can make this molecule, can I make related molecules using the same methodology? What are the limits of this reaction? In Project 4, your class will investigate the scope of the methodology outlined in Figure 2. Each lab group will attempt to prepare a different 4,5-diarylisoxazole by applying the procedure to a different pair of aryl ketone and aryl aldehyde starting materials. If we are successful, Dr. Pollock would like to test the product isoxazoles for activity against various cancer cell lines.
To plan your synthesis, work on Part A of the Project 4 Experiment Design Plan and bring a printed copy to lab. You will continue to work on this plan (and get instructor approval for each step) as you progress through the project. You will have 4 weeks of lab time to complete the reactions, gather data, ask questions, and prepare for your presentation.
Note that the simple example in Figure 2 does not provide any way to distinguish between the two aromatic rings. If either or both of the aromatic rings are substituted, the final product theoretically could be either of two isomeric isoxazoles. As part of your planning process, you should consider how you might determine which of these isomers is generated.
