Project 3: Remarkable Regioselectivity
Simple Synthesis of an Unsymmetrical Pyrrole
Heterocycles are cyclic compounds in which an atom other than carbon is incorporated in the ring. Pyrrole (Figure 1) is one of the most common and biologically important aromatic heterocycles. The pyrrole ring core can be found in a variety of natural products with interesting properties. For example, the structure of pyrrolomycin B is characteristic of a class of pyrrole-containing compounds originally isolated from soil bacteria. These pyrrolomycins have been found to exhibit potent antimicrobial properties. Students paying attention in their biology courses might recognize the structures of heme or chlorophyll, both of which contain four pyrrole or pyrrole-derived subunits. Finally, pyrrole forms the core structure of the cholesterol-lowering drug Lipitor, which earns approximately $2 billion in annual revenue for the pharmaceutical company Pfizer.
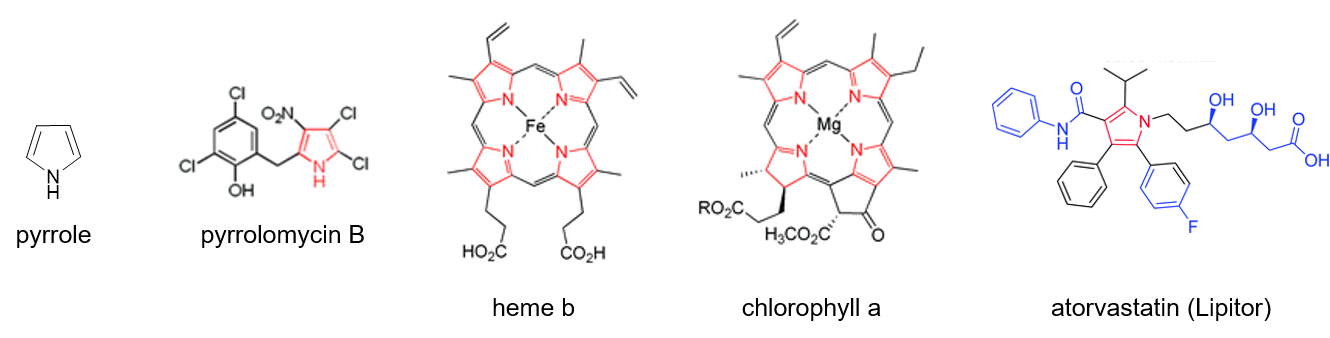
Figure 1. The structure of pyrrole and some pyrrole-containing compounds with interesting properties.
Given their importance, chemists have developed a variety of synthetic methods to generate pyrroles. In 1973, Russian chemist S.I. Zav’yalov reported the pyrrole synthesis outlined in Figure 2. Reaction of the aldehyde component of 1 with the amine functionality of 2 generated imine 3. The direct conversion of imine 3 to pyrrole 5 by reaction with acetic anhydride (4) was unexpected. Despite its simplicity and the use of readily available and non-toxic reagents (especially amino acid 2), this route to pyrroles has remained largely underexploited for over four decades.
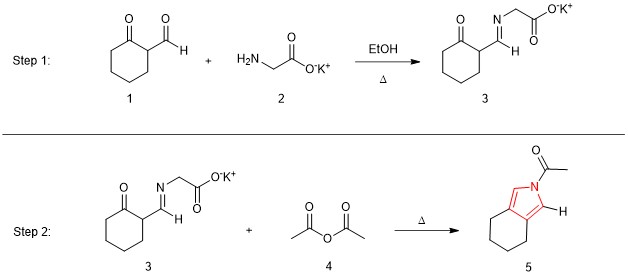
Figure 2. The two-step pyrrole synthesis reported by Zav’yalov in 1973.
In Project 3, you will attempt to apply the Zav’yalov methodology to a relatively simple model system (Figure 3). The two-step pyrrole synthesis begins with the reaction of benzoylacetone (6) with the amino acid glycine (7) under strongly basic conditions. Acidification at the end of the reaction protonates the carboxylic acid group, and you will isolate and characterize the product enamine. Note that reaction could occur at either ketone in 6. In fact, the condensation is highly regioselective, producing only one of the two possible isomers (8 or 9). To determine which isomer is generated, you will learn and apply two new 2D NMR techniques (HSQC and HMBC).
In the second step of the reaction sequence, each potential enamine (8 or 9) would produce a different pyrrole product (10 or 11) upon cyclization under conditions similar to those reported by Zav’yalov (Step 2). You will perform this reaction on your enamine and again utilize 2D NMR data to confirm that the expected pyrrole isomer is produced. In this case, you will have a full set of 2D NMR data (COSY, NOESY, HSQC, HMBC) and you must decide how best to use the combined data to confirm which of the two isomers was generated in your reaction. (Do you need to use all the data? Is the data set internally consistent and a good match for one of the structures but not the other?)
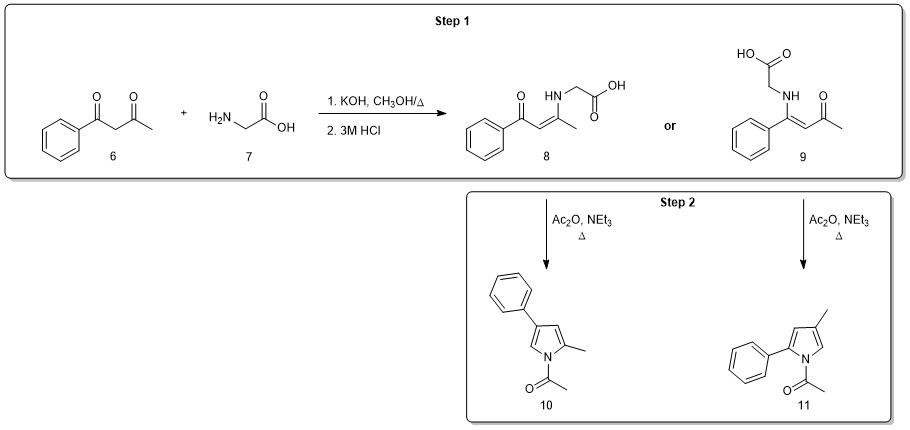
Figure 3. Project 3 reaction scheme with possible products.
