Project 1: Two-Steps Away
Organic chemists often attempt to make novel compounds by applying a generic reaction procedure to specific substrates. This requires them to apply “literature precedent” (the laboratory procedure reported for a different compound) as a template for the reaction on their new substrate(s). In order to complete the two-step synthesis of the Project 1 target compound, you must apply the literature precedent supplied below to the iodovanillin you produced in Part A of this project. The Literature Templates Workshop will help you think through this process.
The role of palladium in organic chemistry has revolutionized the way chemists conduct synthesis. One groundbreaking application of palladium chemistry was developed by Dr. Akira Suzuki of Hokkaido University in 1979. His contribution to synthetic chemistry has come to be known as the “Suzuki Cross-Coupling” method, for which he received the Nobel Prize in 2010. This reaction occurs between an aryl or alkyl halide and an aryl or alkyl boronic acid in the presence of a palladium catalyst and a base (Figure 1). Typically, the reaction involves coupling of an aryl halide with an arylboronic acid (Figure 2). The Suzuki method is frequently utilized in synthesis in both academia and industry.
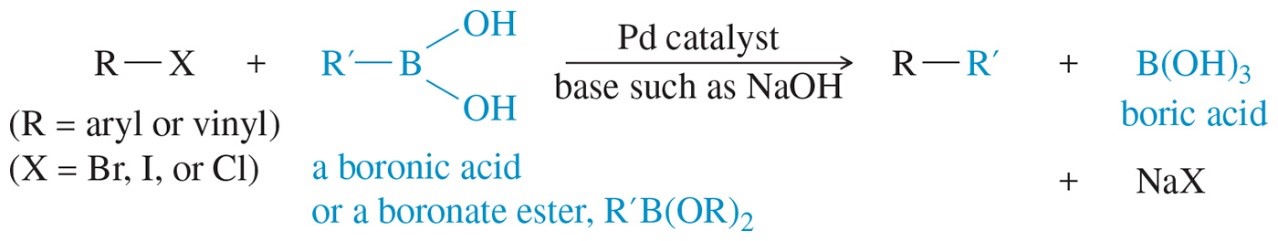
Figure 1 Generic description of the Suzuki Cross-Coupling Reaction
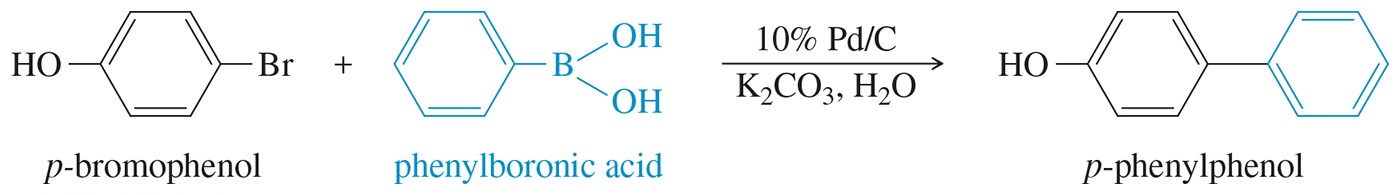
Figure 2 Example of a Suzuki Coupling between aromatic compounds
