Column chromatography is another form of solid-liquid chromatography. In fact, it is just a three-dimensional version of thin layer chromatography (TLC). In column chromatography, a larger quantity of sample is applied to the top of a cylindrical column of adsorbent (stationary phase). Solvent (mobile phase) is then allowed to pass through the column from top to bottom. (See Figure 1 below.)
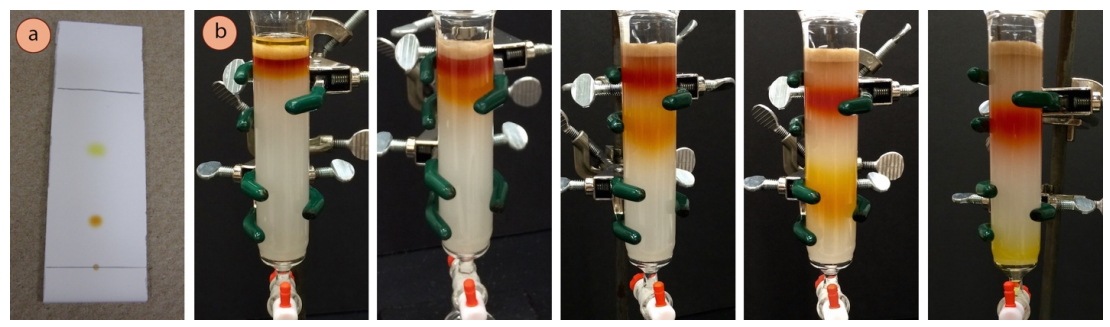
Figure 1. The separation of a mixture by column chromatography. Note that a sample travels UP at TLC plate while it travels DOWN a column. Should more polar compounds reach the bottom of the column faster or slower? (Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_Lab_Techniques_(Nichols)/02%3A_Chromatography/2.04%3A_Column_Chromatography/2.4A%3A_Macroscale_Columns)
As the mobile phase traverses the length of the column, the sample separates by partitioning between the mobile and stationary phases. Ideally, each component of the sample reaches the bottom of column independently. Collecting these individual “fractions” of the sample in separate containers (e.g., in test tubes as illustrated in Figure 2) enables the effective separation of the components of a mixture on a large scale.
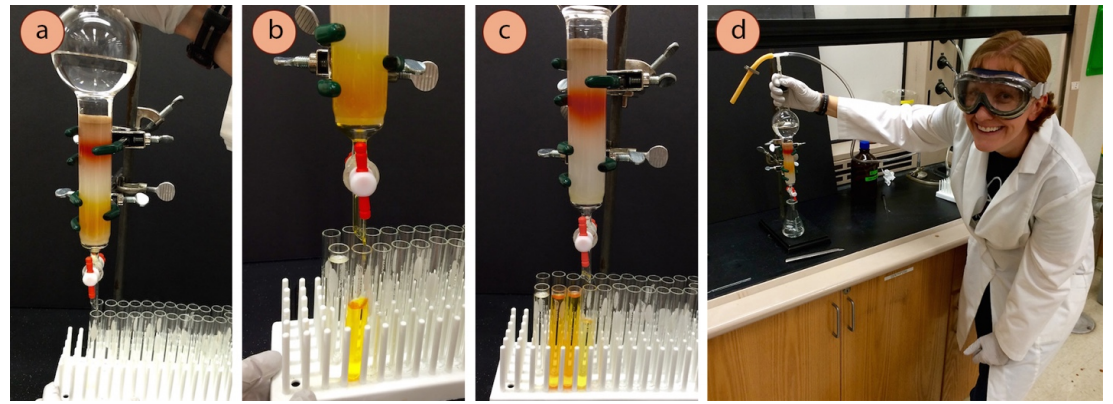
Figure 2. Mixture components separated by column chromatography are collected in separate test tubes. (Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_Lab_Techniques_(Nichols)/02%3A_Chromatography/2.04%3A_Column_Chromatography/2.4A%3A_Macroscale_Columns)
Video: Column Chromatography Setup Example (Small Column)
