Literature Precedent (Apply as necessary for Reaction B)
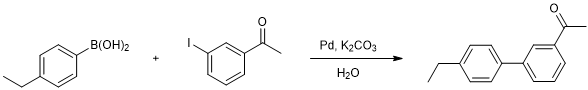
4′-ethyl-3-acetylbiphenyl. A mixture of m-iodoacetophenone (1.0 mmol), p-ethylboronic acid (1.3 mmol), and potassium carbonate (3.2 mmol) in water (20 mL) was treated with 0.319 mL of a 1 mg/mL solution of Pd (in aqueous 5% HCl). The reaction mixture was then heated to reflux for 30 minutes. After the flask was cooled enough to handle, the mixture was gravity filtered to remove any solid palladium particles. Ethyl acetate (15 mL) and 3M HCl (3 mL) were added to the filtrate, which was then swirled to ensure dissolution of any solids. The layers were separated, and the aqueous phase was extracted a second time with ethyl acetate. The combined organic layers were washed with brine and dried over sodium sulfate. The solvent was removed by rotary evaporation to give the crude product. This solid was recrystallized using 9:1 methanol:water to yield a white solid. The product was characterized by GC-MS and ¹H NMR (CDCl3).
Reminders & Hints
1. Don’t forget to fully prepare your Lab Notebook according to the guidelines before entering the lab.
2. Remember that you are NOT performing this procedure as written. You must decide on the correct substrates and quantities to use in the reaction. Your goal should be theoretical yield of 350 mg of target product.
3. You must complete the Project 1B Prelab Quiz before entering the lab to perform this procedure.
