Project 1: Two-Steps Away
You will begin the construction of the Project 1 target compound by iodinating vanillin, a readily available natural product. Electrophilic aromatic substitution (EAS) occurs when an aromatic molecule attacks an electrophile to yield a substituted aromatic ring (Figure 1). If the aromatic ring already contains a substituent, the nature of that substituent will determine the overall reactivity of the aromatic ring and will direct the electrophile to certain location(s) on the ring.

Figure 1. Generic reaction of an aromatic ring with an electrophile
In this experiment, the aromatic compound vanillin will be iodinated (Figure 2). Electrophilic aromatic halogenations typically require the use of an elemental halogen like Cl2 or Br2 in the presence of a Lewis acid catalyst. These elemental halogens, however, can be dangerous to use because of their corrosive nature. Iodine, I2, is less corrosive but also less reactive in electrophilic aromatic substitution reactions. In this experiment, you will use a combination of an easily handled iodide salt (NaI) and the oxidizing agent calcium hypochlorite [Ca(OCl)2] to generate a small amount of I2 (or more likely I+) in the reaction vessel. This species will then react with an aromatic ring via the typical EAS mechanism.
A procedure written in the style you might find in a journal article is provided for you. However, you must “scale” the quantities of materials used in the experiment such the theoretical yield of your reaction is 2.0 grams. The Reaction Scaling Workshop (on Blackboard) will help guide you through this process.
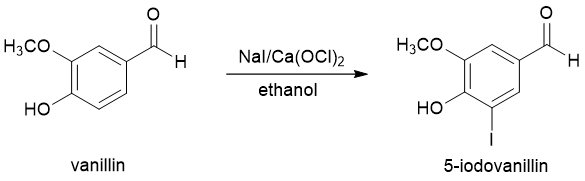
Figure 2. Iodination of vanillin
