Project 2: It’s All Conditional
The Influence of Conditions on a Reaction Outcome
The outcome of a chemical reaction may depend on a variety of factors such as temperature, solvent, pH, reactant ratios, exposure to light, etc. In Project 2, you will compare the products formed by the reaction of p-methoxyacetophenone (PMA) with household bleach under two sets of conditions.
Under the influence of mild heat, bleach is known to oxidize the ketone functional group in PMA to the corresponding carboxylic acid (Figure 1).
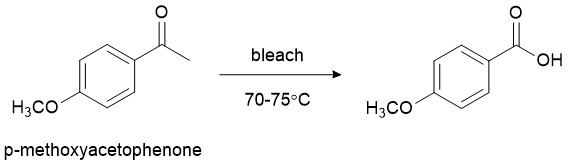
Figure 1. When heated, p-methoxyacetophenone (PMA) reacts with bleach to form a carboxylic acid.
The active ingredient in household bleach is sodium hypochlorite (NaOCl), an oxidizing agent. Remember that in organic compounds, oxidation can generally be recognized as either 1) an increase in the number of bonds between carbon atoms and electronegative atoms (usually oxygen or halogens) or 2) a decrease in the number of bonds between carbon and hydrogen (Figure 2).
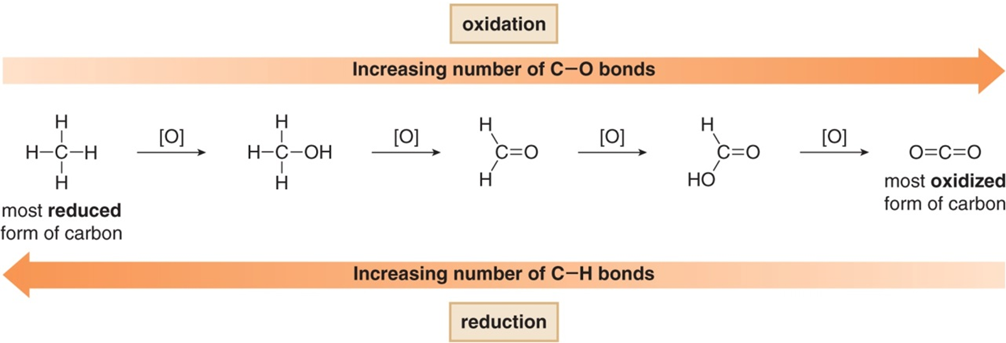
Figure 2. Oxidation reactions result in an increase in the number of bonds between carbon and oxygen (or some other element more electronegative than carbon).
In an aqueous solution such as bleach, the hypochlorite ion is involved in two important equilibria. The first (Equilibrium A, Figure 3) is an acid-base reaction with water to produce hypochlorous acid. The second (Equilibrium B, Figure 3) is a reaction with aqueous chloride anions (present in the bleach solution) to generate molecular chlorine, which is also a powerful oxidizing agent. An analysis of the species present in these two equations should suggest that the reaction pH might strongly influence the concentration of the two potential oxidizing agents and therefore the structure of the product generated by the reaction of bleach with the organic substrate.
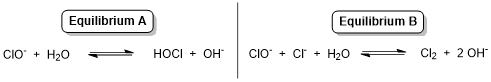
Figure 3. The hypochlorite anion (ClO-) in bleach participates in two important equilibria.
You will test the idea that pH can influence the course of the PMA/bleach reaction by determining the structure of the product generated in the presence of acetic acid. As you plan your lab work, review any techniques with which you are uncomfortable. Remember that descriptions and generic procedural details for basic lab techniques are available in the Lab Techniques section.
Whenever you are performing a chemical reaction in which the product structure is unknown, it is helpful to analyze the characterization data (e.g., MS, IR, NMR) of the substrate (reactant molecule) before running the reaction. You may then be able to spot what changed about the molecular structure by comparing the product data to that of the reactant. Remember that two databases you may use to look up standard spectra of many organic compounds, including p-methoxyacetophenone, are linked here.

One response to “Background 2 – Archive”