Vacuum filtration, which uses low pressure to draw the liquid phase of a mixture through the filtering medium, provides a more effective separation of solids and liquids. The rapid airflow through the solids retained in the funnel also helps dry them rapidly. Vacuum filtration is most commonly used when the primary objective is the isolation of relatively large quantities of purified, dry solids.
The apparatus for vacuum filtration is a perforated funnel (e.g., Buchner or Hirsch funnels) connected by an airtight stopper or adapter to a heavy-walled filter flask (Figure 1). In order to retain the solids, an appropriately sized piece of circular filter paper must be laid flat on the bed of the funnel to completely cover the perforations without curling up the sides of the funnel.
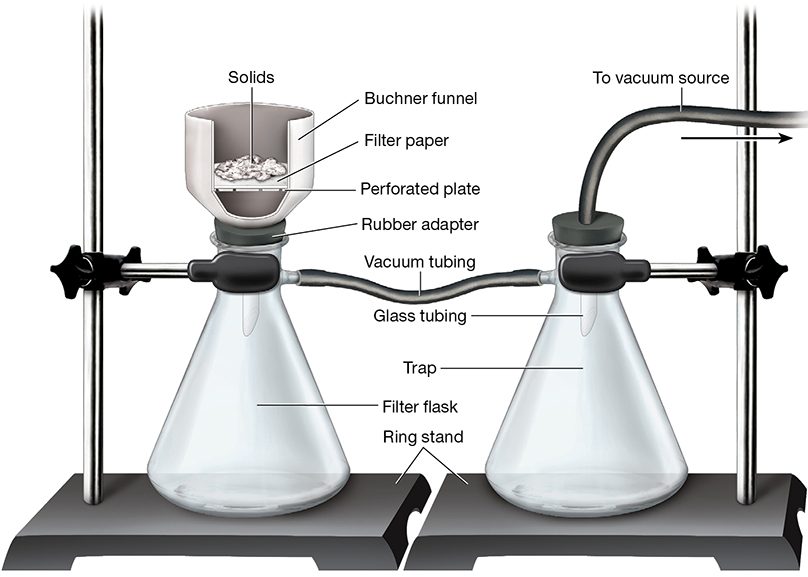
Figure 1. Apparatus for vacuum filtration. The optional trap prevents backflow from a water aspirator into the filter flask and is especially important if the liquid phase will be collected and used.
The sidearm on the filter flask is the attachment point for a tube that is connected to a vacuum source. When the vacuum is applied and the solid/liquid mixture is added to the funnel, the low pressure inside the flask causes the liquid to be forced rapidly through the filter paper while the solids remain in the funnel.
In some cases, a filter trap may be included between the filter flask and the aspirator inlet to catch any water backflow and prevent it from entering the filter flask. The trap is often omitted when the filtrate will be discarded. The solids retained during a vacuum filtration should be washed with a small volume of the appropriate solvent (typically the same liquid removed during the filtration) in order to remove any residual impurities. To reduce the chances of dissolving the solid, the wash solvent is usually chilled.
After washing, the solids are allowed to sit in the funnel with the vacuum running for several minutes to help them air-dry. They are then removed from the funnel by inverting it over a piece of weigh paper or a watch glass. The solids and the filter paper should fall out, but a microspatula may be used to help remove the filter paper as necessary.
Forceps are then used to pick up the wet filter paper, and the microspatula can be used to gently scrape off any remaining solids into the pile. The solids are completely dried by allowing them to sit while the residual liquid evaporates, or they may be further dried by the application of heat (e.g., in an oven).
General Protocol
1. Set up the vacuum filtration apparatus (Figure 1) by clamping a filter flask to a ring stand. Fit the flask with a Buchner funnel and rubber stopper or adapter. Attach a vacuum hose from the inlet on the vacuum source (or filter trap, if applicable).
2. Place an appropriately sized piece of filter paper flat in the funnel. Ensure that all the perforations are covered and that the paper does not curl up the sides.
3. Wet the filter paper with the appropriate solvent (typically a fresh sample of the same liquid that will be removed during the filtration).
4. Turn on the vacuum, which should seal the paper to the bottom of the funnel.
5. Pour the mixture to be filtered into the funnel to begin the filtration.
6. Once the liquid has been removed, wash the solids with the appropriate solvent.
7. Allow the solids to dry in the filter with the vacuum on for at least 10 minutes.
8. Discontinue the vacuum by turning the valve 180º.
9. The solids may be recovered by inverting the Buchner funnel on a watch glass or piece of weigh paper. Use a microspatula to help you remove the piece of filter paper from the funnel. Using forceps, pick up the filter paper and gently scrape off any remaining solids with the microspatula.
Video: Vacuum Filtration Setup in the UR Organic Chemistry Labs
This video demonstrates the setup for vacuum filtration in the UR Organic Chemistry Labs.
