Background
Many alkene addition reactions proceed through mechanisms that lead to specific stereochemical consequences. For example, halogenation of an alkene takes place through anti addition (Figure 1). In this experiment, you will brominate trans-cinnamic acid and use your data to confirm the stereochemical outcome of the reaction.
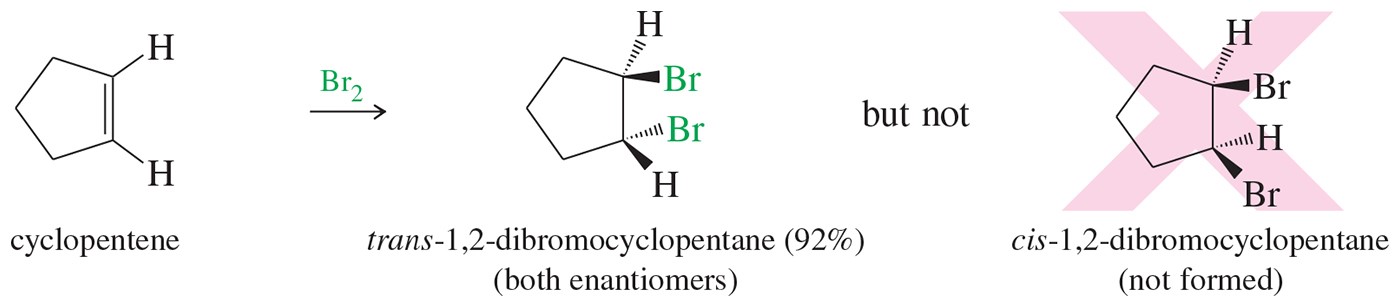
Figure1. Halogenation of alkenes proceeds with anti addition of the halogen atoms.
In this project, you will halogenate an alkene without using hazardous liquid Br2. Instead, this species is introduced through the reagent pyridinium tribromide, which is a more safely handled solid. The equilibrium illustrated in Figure 2 provides for a “slow release” of bromine into the reaction medium.

Figure 2. Equilibrium between pyridinium tribromide and elemental bromine.
The specific reaction you will perform is illustrated in Figure 3. The product (2,3-dibromo-3-phenylpropanoic acid) has two stereocenters, and four stereoisomers could potentially be generated by the reaction. Using your knowledge of halogenation reactions, you should predict which of these isomers is likely to form. Melting point analysis will allow you to confirm the stereochemistry of the reaction products: The (2R, 3S) and (2S, 3R) enantiomers have a melting point of 204°C while the (2R, 3R) and (2S, 3S) enantiomers have a melting point of 95°C.
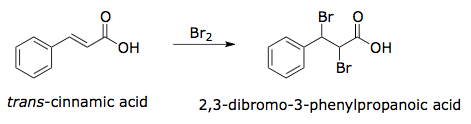
Figure 3. Overall reaction for the bromination of trans-cinnamic acid.
Procedure
1. In a 250 mL round bottom flask, stir cinnamic acid (0.017 mol) with 60 mL of glacial acetic acid at room temperature until the solution becomes homogeneous.
2. Add Pyridinium tribromide (0.020 mol) to the reaction mixture, and heat the solution to reflux for 20 minutes.
3. After the reflux period, cool the solution to room temperature, then pour it into a 250 mL Erlenmeyer flask.
4. Add distilled water (75 mL) and place the flask in an ice-water bath for 15 min to promote crystallization of the product.
5. Collect the solid dibromide by vacuum filtration and wash it with cold distilled water until no yellow color is apparent. Allow the solid to dry on the funnel for at least 30 minutes.
6. Determine the product yield and characterize it by melting point analysis, IR, and NMR (d6-acetone).
