Project 2: In Search of FAME
Methanolysis of Trimyristin: An Introduction to Chemical Reactions & Analysis
Introduction
Trimyrisitin is an example of a triacylglycerol, an important class of biomolecules commonly referred to as “triglycerides.” They are the most abundant lipids and the primary form of stored chemical energy in the body. Figure 1 diagrams the generic structure of a triacylglycerol and provides an example structure. Importantly, the triacylglycerol structure is dominated by three ester functional groups, the bonds of which join a glycerol portion to three separate fatty acid portions. Note that the fatty acid portions of a triglyceride (illustrated in blue) are not necessarily identical to each other.
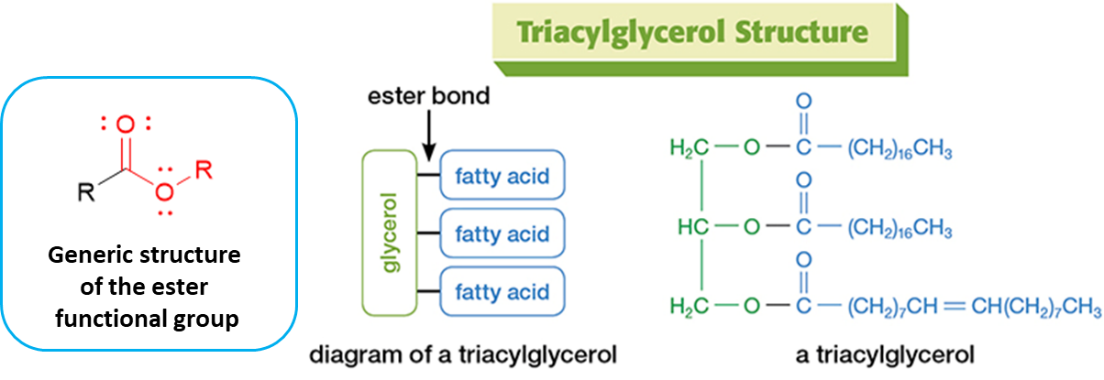
Figure 1. Overview of triacylglycerol structural features
Transesterification is a chemical reaction in which the alkoxy portion of an ester (e.g., -OR2 in Figure 2) is exchanged for another alkoxy group, typically derived from an alcohol used as the reaction solvent (e.g., -OCH3 in Figure 2). The reaction is catalyzed (i.e., sped up) by adding either acid or base.
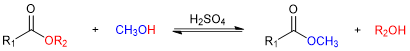
Figure 2. A generic transesterification reaction using methanol (CH3OH).
In trimyristin and all other triacylglycerols, the alkoxy portion (-OR2) of each of the three esters is part of the same glycerol backbone. Unlike many other triglycerides, however, the three fatty acid portions of trimyristin are identical. Transesterification of trimyristin with methanol (CH3OH) is therefore expected to generate only the two products illustrated in Figure 3. (Note: This equation is not balanced. That will be part of your job in the Reaction Preparation Workshop!).
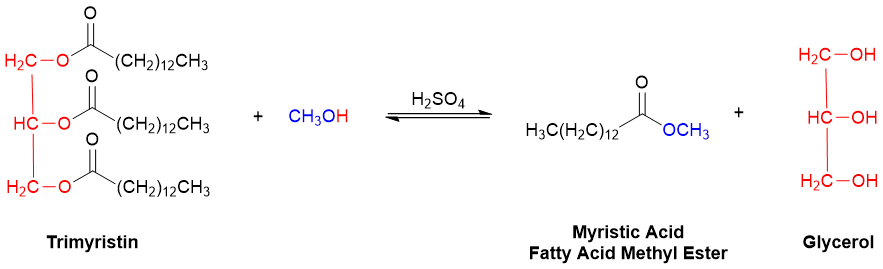
Figure 3. The transesterification of trimyristin with methanol (CH3OH).
Project 2 Objective
Your task in this Project is to perform this transesterification of trimyristin to generate the fatty acid methyl ester (FAME) of myristic acid (a.k.a, methyl myristate). You will analyze the success of your chemical reaction in terms of the quantity, identity, and purity of the isolated product. Note that methyl myristate is not a solid, so melting point analysis cannot be used. In addition, the structural similarity between methyl myristate and trimyristin means that IR spectroscopy is of little utility in distinguishing between the two species. For these reasons, Project 2 also introduces two new methods of product analysis: gas chromatography and mass spectrometry.
