Background
Alkenes can be converted to epoxides by various oxidizing agents such as mCPBA (Figure 1). The reaction takes place in a single step so the geometry of the π bond is retained in the product. In this project, you will perform an epoxidation and use experimental data to verify that the reaction is truly stereospecific.
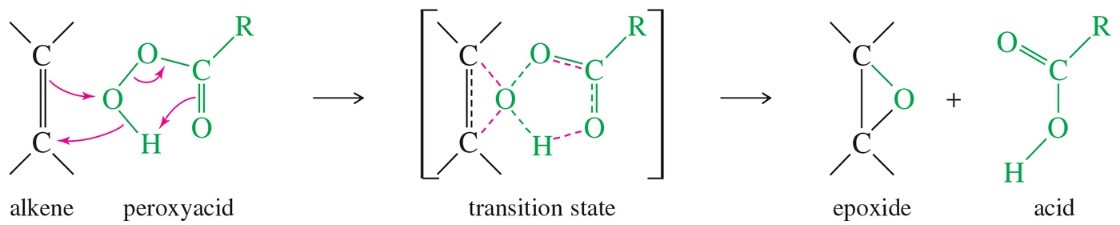
Figure 1. Generic mechanism of alkene epoxidation with a peroxyacid such as mCPBA.
Many common reagents used for epoxidation reactions, especially peroxides, decompose rapidly and can be hazardous to store for long periods of time. To avoid this problem, you will generate the active reagent, dimethyldioxirane, in situ (meaning “in the reaction flask”) by mixing Oxone (a safely handled oxidizing agent) with acetone. The dimethyloxirane is very reactive, so the order of addition is important for this reaction. The reaction also generates acidic KHSO4 as a byproduct, so the mixture includes aqueous NaHCO3 to maintain the optimum reaction pH (~7-9).
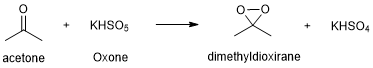
Figure 2. The active epoxidation reagent (dimethyldioxirane) is generated in situ.
In this investigation, you will make the epoxide of trans-anethole, a natural product that gives the characteristic flavor to anise, fennel, and liquorice (Figure 3). As usual, you will assess the outcome in terms of yield, purity, and identity of the reaction product.
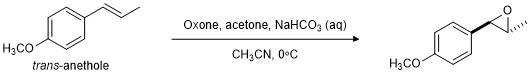
Figure 3. Epoxidation of trans-anethole.
Recall that coupling constants in 1H NMR spectra depend in part on the angle between the protons. Due to the rigid nature of the epoxide ring, this angle-dependence of the coupling constant (J) makes it possible to distinguish between cis and trans epoxides (Figure 4).
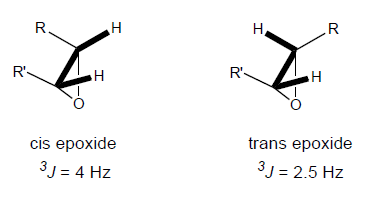
Figure 4. Characteristic coupling constants between cis and trans protons on an epoxide
Keep in mind that the protons on the epoxide ring may not couple only to each other, so the splitting pattern might be somewhat complex for certain signals in the product spectrum. It may be helpful to review this video on complex coupling from NMR Workshop III.
Procedure
1. Dissolve anethole (2 mmol) in a 1:1 mixture of acetone and acetonitrile (20 mL).
2. Add a saturated solution of sodium bicarbonate (10 mL), then chill the reaction mixture in an ice-water bath for 10 minutes.
3. Add a mixture of oxone (4.4 mmol) in water (10 mL), and stir the reaction mixture at 0°C for 1.5 hours. NOTE: The molecular weight of oxone is 307.38 g/mol.
4. Add water (20 mL) to the reaction mixture, and extract the resulting solution with ethyl acetate (2 × 30 mL).
5. Wash the combined organic layers with brine (50 mL), dry over sodium sulfate, and gravity filter to give a clear solution.
6. Remove the solvent by rotary evaporation to give the product as a clear, colorless oil.
7. Determine the product yield and characterize it by GC-MS, IR, and NMR.
