Background
In this project, you will perform a simple chemical reaction that converts the compound 9-fluorenone into 9-fluorenol. The balanced chemical equation for this reaction is given in Figure 1.
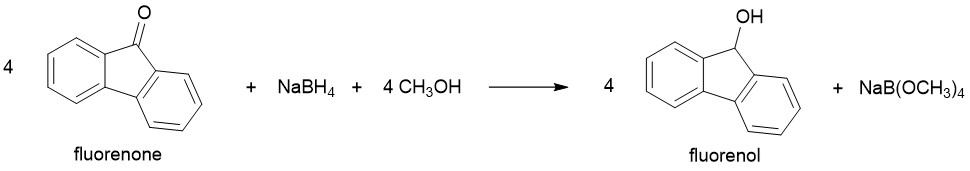
Figure 1. The reduction of 9-fluorenone with sodium borohydride.
Notice that the substrate (9-fluorenone) contains a ketone functional group that is transformed into an alcohol functional group in the product (9-fluorenol). This change is an example of a reduction, and the sodium borohydride (NaBH4) serves as the reducing agent in the reaction. In organic compounds, a reduction can generally be recognized as either 1) an increase in the number of bonds between a carbon atom and hydrogen or 2) a decrease in the number of bonds between a carbon and a more electronegative atom (usually oxygen or halogens). This concept is illustrated in Figure 2.
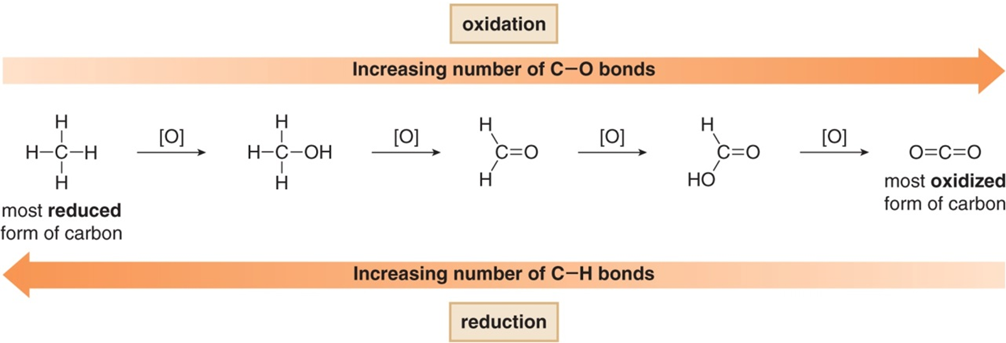
Figure 2. Reduction reactions commonly result in an increase in the number of bonds between carbon and hydrogen.
This pattern allows organic chemists to classify the various functional groups into different “oxidation states.” The alcohol functional group is in a lower oxidation state (i.e. “less oxidized”) than the ketone functional group. If you take CHEM 206, you will learn a lot more about these types of reactions!
Procedure
1. In a 125 mL Erlenmeyer flask, dissolve fluorenone (5.5 mmol) in methanol (10 mL). If the solid does not dissolve immediately, it may be necessary to warm the mixture gently on a hotplate for a few minutes. Allow the solution to cool to room temperature before proceeding to the next step.
2. Add sodium borohydride (2.2 mmol) to the reaction mixture. Allow the reaction to proceed at room temperature, swirling the flask occasionally.
3. In 10 minute intervals, monitor the reaction by thin layer chromatography (TLC) using 8:2 hexane:ethyl acetate as the mobile phase. When you perform your TLCs, you want to compare the behavior of the reaction mixture to that of the starting material (fluorenone). You can spot both samples on the same plate. To prepare a standard sample of fluorenone, dissolve a half-rice-grain-sized amount in ~2 mL acetone.
4. When you judge the reaction to be complete (by TLC), add 1 M hydrochloric acid (1 mL) to the reaction mixture.
5. Add ~20 mL ethyl acetate and ~20 mL distilled water to the reaction mixture. Swirl the flask to dissolve any solids that have formed.
6. Transfer the mixture to a separatory funnel, and separate the two layers. Save the organic layer in a 50 mL Erlenmeyer flask, and return the aqueous layer to the separatory funnel.
7. Extract the aqueous layer with additional ethyl acetate (~20 mL).
8. Combine the two organic layers and wash them with brine (~20 mL).
9. Transfer the organic layer to a 125 mL Erlenmeyer flask and dry it over sodium sulfate.
10. Gravity filter the mixture into a 100 mL round bottom flask.
11. Remove the solvent by rotary evaporation.
12. Recrystallize the solid product using 75:25 methanol:water. [Add hot solvent to the round bottom flask to dissolve and loosen some of the solids. Transfer the solid/liquid mixture to an Erlenmeyer flask to complete the recrystallization as usual.]
13. Collect the purified solid by vacuum filtration, allowing it to dry in the Buchner funnel for 30 minutes.
14. Determine the yield of the product and analyze it by melting point analysis, GC-MS, IR, and NMR.

One response to “Project 5A – S23”