Background
In this project, you will explore the reactivity of aromatic compounds such as p-methoxyacetophenone (PMA). As you have learned in CHEM 205, the aromatic ring does not exhibit reactivity typical of aliphatic alkenes. You will employ the structure determination skills you learned this semester to determine the product generated by the reaction of PMA with bleach (Figure 1).
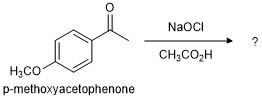
Whenever you are performing a chemical reaction in which the product structure is unknown, it is helpful to analyze the characterization data (e.g., MS, IR, NMR) of the substrate (reactant molecule) before running the reaction. You may then be able to spot what changed about the molecular structure by comparing the product data to that of the reactant. Remember that two databases you may use to look up standard spectra of many organic compounds, including p-methoxyacetophenone, are linked here.
Procedure
1. In a 25 mL round bottom flask, combine a small stir bar, p-methoxyacetophenone (0.407 g) and glacial acetic acid (3.0 mL).
2. While stirring the mixture on a stir plate, add household bleach (5.5 mL) dropwise.
3. Stir the resulting solution at room temperature for 30 min.
4. Add an additional 2.0 mL of bleach allow the reaction to stir for another 20 minutes.
5. Add distilled water (15 mL) to the reaction flask. A solid should precipitate.
6. Collect the precipitate by vacuum filtration and wash it with additional distilled water.
7. Recrystallize the solid using 75:25 methanol:water.
7. Dry the crystals on the Buchner funnel for at least 30 min.
8. Determine the product yield and characterize it by melting point analysis, GC-MS, IR, and NMR.
