Project 3: What’s That Smell?
Isolation of Carvone by Column Chromatography of Spearmint Oil
Introduction
Spearmint oil is a natural product obtained by steam distillation of volatile compounds from the flowering tops of the plant Menta spicata. It is used as a flavoring or scent agent in a variety of products, such as toothpaste, lip balm, and mouthwash. The oil is a mixture with three primary constituents: (+)-limonene, (-)-carvone, and (1R, 2R, 4R)-dihydrocarveol (Figure 1). The most abundant component, (-)-carvone, is responsible for the characteristic odor and favor of spearmint.
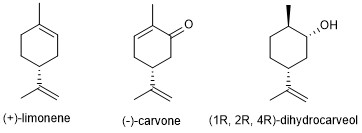
Figure 1. Major components of spearmint oil.
Project 3 Objective
Your objective in Project 3 is to isolate a pure sample of (-)-carvone from spearmint oil. To accomplish this task, you will learn to use two new chromatographic techniques: Thin Layer Chromatography (TLC) and Column Chromatography (Figure 2). TLC is primarily an analytical tool used on small amounts of material to determine the contents of a sample. Column chromatography is a larger-scale technique used to purify bulk quantities of material.
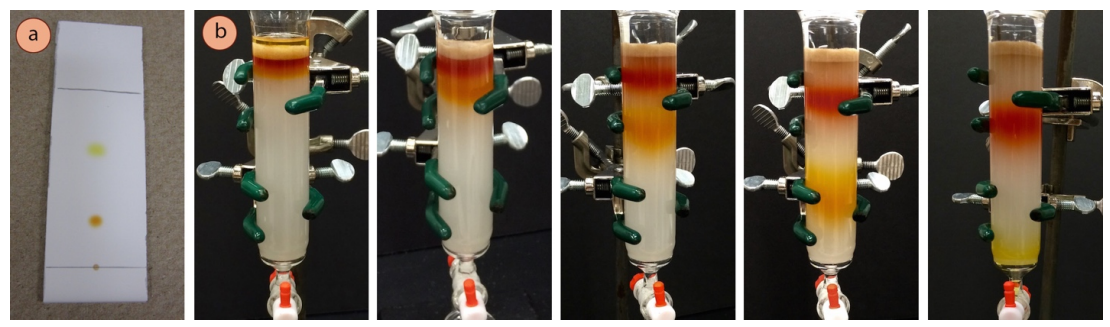
Figure 2. A TLC experiment (a) is an analytical technique used to determine the number/identity of components in a sample. Column chromatography (b) separates the components of a mixture as they pass through the column at different rates. Note that a sample travels UP at TLC plate while it travels DOWN a column. (Source:https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols))
You will begin by using TLC to analyze the spearmint oil. Comparison to three standards (limonene, carvone, and dihydrocarveol) will allow you to identify the spearmint oil components on your TLC plates. In addition, you will determine the best solvent system for separating the mixture from three options: 9:1 Hexane:Ethyl Acetate; Hexane; and Ethyl Acetate.
After TLC analysis, you will subject 1.5 mL of spearmint oil to column chromatography, isolate the carvone by recombining fractions that contain only that compound, and then report the mass/volume percent of carvone isolated from your sample. Finally, you will use GC-MS, IR, and ¹³C NMR data to characterize your isolated product and confirm its identity and purity.
