Project 1: That’s Fat!
Isolation of Trimyristin:
An Introduction to Lab Work & Molecular Characterization
Introduction
Many important medications and other natural products were first discovered by extraction from their natural sources. Extraction is the selective dissolution of one substance in a mixture into a separate phase. There are a variety of extraction techniques that may be used to achieve different goals, and these are generally labeled by referencing the two interacting phases. For example, solid-liquid extraction involves the interaction of a liquid phase with a solid phase. You are probably familiar with this technique, which is used to make coffee (and tea): Hot water is used to extract caffeine and various flavor compounds in solid coffee grinds into a liquid phase. Chemists would consider the coffee drink an “extract” (Figure 1).
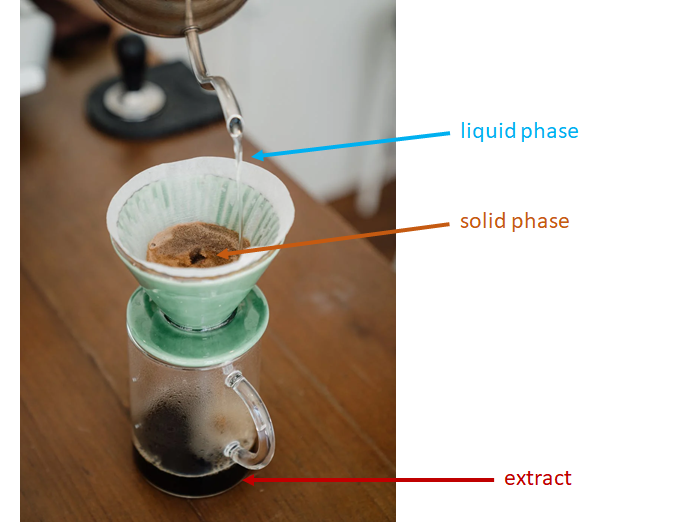
Figure 1. Making coffee is an example of solid-liquid extraction.
Watch this video to learn more about the chemical concepts involved in extraction. (You must be on campus or connected to the UR network by VPN to access this material). The theory is covered in the first 3 minutes of the video. You do not need to worry about understanding the specific experiments covered in the rest of the video, but watching the entire video is a good preview of some types of work done in an organic chemistry lab.
Project 1 Objective
In Project 1, you will extract and purify trimyristin (Figure 2). This compound is an example of a naturally occurring triglyceride (i.e., “fat”) found in high concentrations within nutmeg, a spice obtained from the seeds of the East Indian evergreen tree Myristica fragrans.
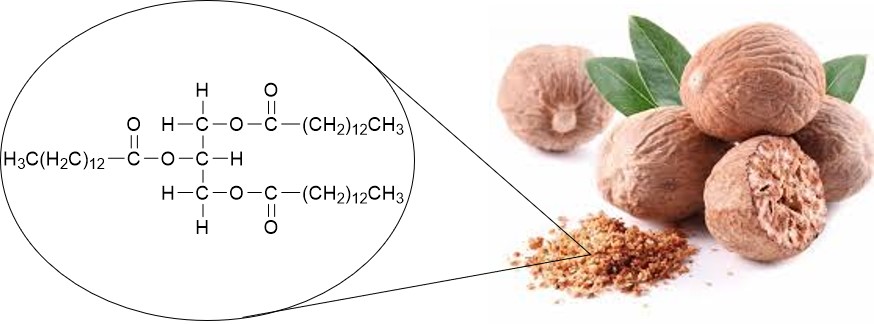
Figure 2. Trimyristin (illustrated here) is easily extracted from nutmeg.
You will obtain a crude sample of trimyristin by a procedure that involves solid-liquid extraction of the nutmeg with a solvent mixture. The isolated solid will then be further purified to yield essentially pure trimyristin. After you have the purified trimyristin in hand, you will obtain a melting point and infrared spectrum to help you verify that the substance is in fact trimyristin.
