Cancer is the leading cause of death worldwide. Thanks to advances in medicine, deaths from communicable diseases have decreased, and life expectancy has increased. However, with people living longer, there is a greater chance of developing noncommunicable diseases like cancer in their lifetime. So, despite medical progress, why are people still dying from cancer?
Cancer is the uncontrolled growth of abnormal cells, and there are hundreds of types, each requiring different treatment approaches. Its ability to mutate quickly makes it especially difficult to treat. Standard cancer treatments include surgery, radiation therapy, immunotherapy, hormone therapy, and one of the most widely used chemotherapy.
Chemotherapy uses drugs to kill or slow the growth of cancer cells. While effective, it also harms healthy cells, leading to a range of side effects. This has driven search for developing new and improved treatments. One promising area of research is metal-based chemotherapy.
The Discovery of Cisplatin
Cisplatin was the first metal-based anticancer drug, discovered in the 1960s and approved by the FDA in 1978. This compound is a platinum (Pt) based drug bound to two chloride (Cl) ions and two ammonia (NH3) groups in a square planar geometry. The arrangement of the groups bound to platinum is extremely important as the cis isomer, where the chlorides are next to each other, exhibits anticancer properties while the trans isomer, where the chlorides are across from each other, does not.
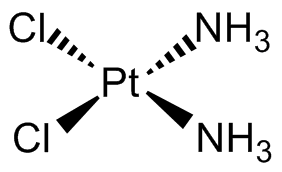
Once inside the body, cisplatin becomes activated when the chloride ions are replaced by water molecules. The activated drug binds to DNA, damaging it and triggering cell death. It has also been found that cisplatin could produce reactive oxygen species (ROS), which also leads to cell death. While extremely effective at killing cancer cells, cisplatin has several side effects, including many toxicities, and emerging resistance. This has led to the development of other platinum-based drugs like carboplatin, known for having fewer side effects. The success of cisplatin opened the door to exploring other transition metals for cancer treatment.
Why Transition Metals?
Transition metals have unique properties that make them ideal for drug development. They can exist in multiple oxidation states, giving them reduction-oxidation (redox) potential. They also bind to a variety of ligands, molecules that attach to the metal, making it possible to design a wide range of compounds with different pharmacological properties. As seen with cisplatin, even changes such as reducing the metal and substituting or releasing ligands can significantly impact how the drug behaves and interacts with cells.
Light-Activated Prodrugs
Developing drugs with improved efficiency and safety requires increased selectivity to cancerous cells and reduced toxicity to healthy cells. An effective approach is developing prodrug treatments, such as photodynamic therapy (PDT) and photoactivated chemotherapy (PACT), which both utilize light-activated prodrugs. Prodrugs are compounds that have little to no pharmacological activity, in this case toxicity, until they have become activated. By delivering light at a specific wavelength to the targeted area only the compound in the spot treated with light will become toxic, so there is minimal damage to healthy cells.
PDT prodrugs transfers electrons or energy to dioxygen (O2) when exposed to light producing ROS which creates oxidative stress and eventually cell death. PDT works best in well oxygenated tissue in order to develop more ROS to kill more cancer cells. PACT prodrugs work by a different mechanism. When activated the PACT prodrug will release a ligand so that the metal component or newly released ligand are now toxic and kill the cancer cells.
Ruthenium Compounds
One of the more studied metals for chemotherapy drugs is ruthenium (Ru). Ru(III) is less toxic and more selective than platinum. It can mimic iron (Fe), allowing it to bind to transferrin, the protein that transports iron into cells. Since cancer cells have a higher demand for iron, this mechanism helps ruthenium compounds more easily enter tumor cells. Under physiological conditions ruthenium has two stable oxidation states Ru(III) and Ru(II), which is more reactive. Once reduced, Ru(II) can bind to DNA or proteins, ultimately killing the cell. This reduction can even be triggered by light, making ruthenium an ideal candidate for PACT.
Cobalt Compound
Another less studied potential metal for chemotherapy drugs is cobalt (Co). Similar to ruthenium, cobalt has two stable oxidation states under physiological conditions. Co(III) can be reduced to Co(II), a more toxic form. While its exact mechanism of action is still under investigation, cobalt holds potential as a metal-based chemotherapy.
The Future
In our lab, I am testing ruthenium- and cobalt-based prodrugs as potential chemotherapy agents. These compounds aim to be more selective and less toxic, making them a more effective and safer way to treat cancer. The continued development of metal-based chemotherapy drugs could be revolutionary for cancer treatment.
Vienna Tombline
Class of 2027
Chemistry Major
Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A. K.; Pathak, S.; A review on metal complexes and its anti-cancer activities: Recent updates from in vivo studies. Biomedicine & Pharmacotherapy 2024, 171, 116211. https://doi.org/10.1016/j.biopha.2024.116211.
Casini, A.; Pöthig, A.; Metals in Cancer Research: Beyond Platinum Metallodrugs. ACS Central Science 2024, 10 (2), 242-250. https://doi.org/10.1021/acscentsci.3c01340
Graf, N.; Lippard, S. J.; Redox activation of metal-based prodrugs as a strategy for drug delivery. Advanced Drug Delivery Reviews. 2012, 64 (11), 993-1004. https://doi.org/10.1016/j.addr.2012.01.007.
Lee, S. Y.; Kim, C. Y.; Nam, T.; Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Design, Development and Therapy. 2020, 14, 5375–5392. https://doi.org/10.2147/DDDT.S275007
Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. https://doi.org/10.3390/molecules27196485
Ndagi, U.; Mhlongo, N.; Soliman, ME.; Metal complexes in cancer therapy – an update from drug design perspective. Drug Des Devel Ther. 2017, 11, 599-616. https://doi.org/10.2147/DDDT.S119488
Unavane, S.; Patil, R.; Syed, S.; Jain, H. K.; Exploring the therapeutic potential of copper and cobalt complexes as anticancer agents: a comprehensive review. Transit Met Chem 2025. https://doi.org/10.1007/s11243-024-00630-6

Recent Comments