How do proteins ‘know’ what a cell requires of them? This enigmatic knowledge lies in a microscopic relay race inside our cells, called signal transduction. Effectively working as a molecular baton pass, signal transduction starts with a protein that binds to a receptor which affects another protein, affecting another, that ultimately results in the intended effect induced by the signal in the cell.
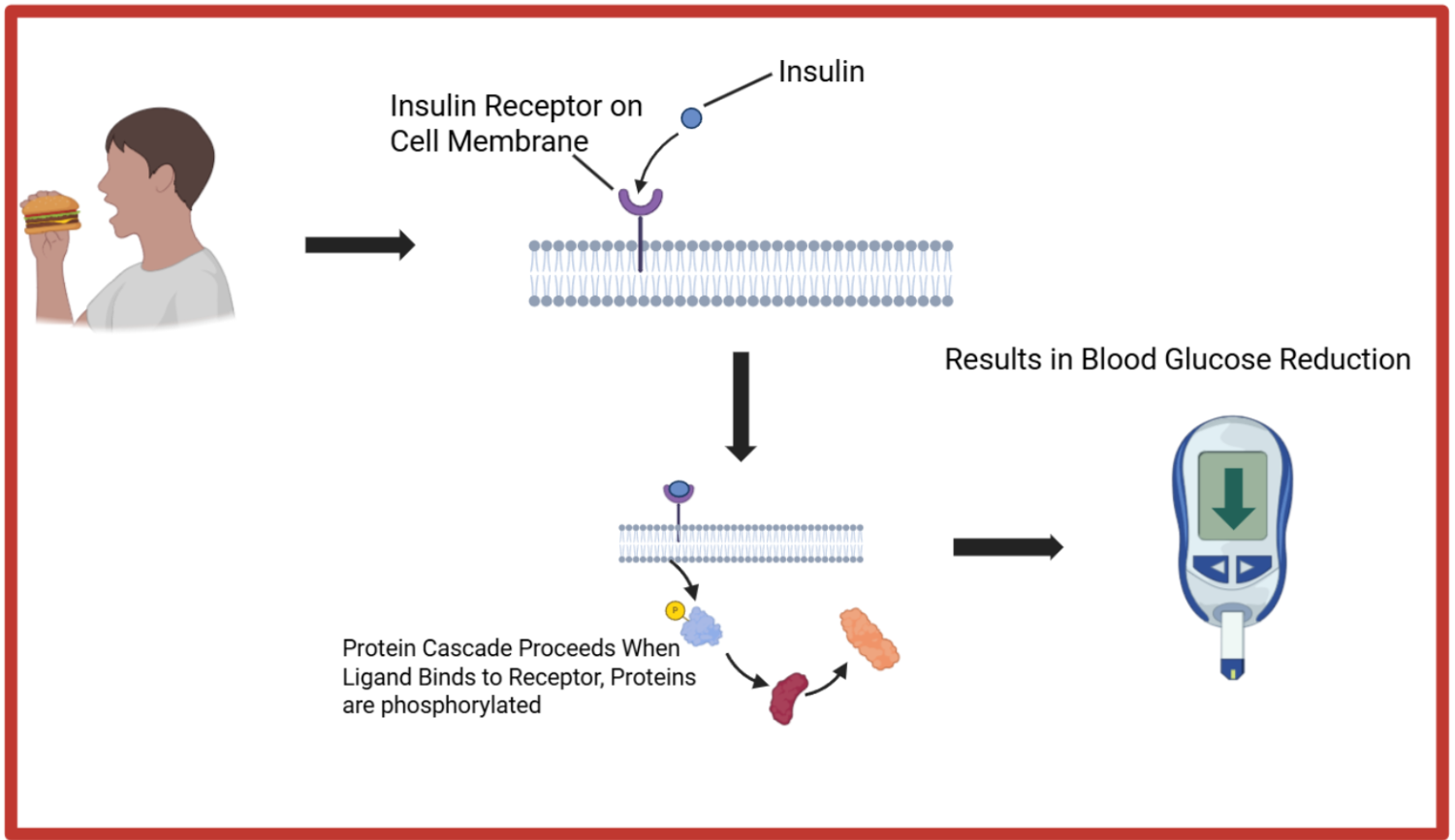
This allows a small chemical signal to multiply and create significant effects, including the production of new proteins. These changes often have lasting impacts on the cell and are essential for its function. A well-known example is insulin. When you eat, insulin is produced and binds to a receptor on the cell’s surface, triggering a signal transduction pathway. Proteins relay the message through the pathway, altering their shapes or adding chemical markers to one another, until the final proteins perform their intended tasks. In this case, opening channels for sugar to enter the cell, which regulates blood sugar levels in the body.
Without signal transduction, cells would be unable to coordinate these vital changes and would be incapable of performing many essential functions. This communication network allows cells to grow, heal wounds, fight infections, and respond to threats, all because proteins ‘know’ exactly what to do.
What Is Signal Transduction?
Signal transduction is how cells sense and respond to changes in their environment by passing a signal to induce activity inside the cell. It starts with a signal, usually a molecule like a hormone, growth factor, or neurotransmitter. This signal acts as a key that binds to a receptor which is a lock. When the key fits, it unlocks a response within the cell, triggering a cascade of consequences.
Once unlocked, the receptor switches on other molecules inside the cell, setting off a chain reaction called a protein cascade. In this cascade, proteins activate other proteins in a specific order, amplifying the original message. Ultimately, the signal reaches its destination and instructs the cell to divide, make new proteins, move, or even self-destruct.
Phosphorylation
Inside the cell, messenger proteins pass the signal along. A common example is a protein kinase, which works by adding a small chemical tag called a phosphate group to other proteins. This allows our metaphorical ‘baton’ to continue to be passed, allowing the signal to continue to be passed along. Many proteins only become active when phosphorylated, acting like a switch that turns them ‘on’ or ‘off’. This careful relay ensures that the right proteins perform the right jobs at the right time. Some proteins might open channels to let nutrients in; others might start building molecules vital for the cell’s function. If even one protein in the chain fails, the entire cascade can break down.
My Own Research and Why Studying Cellular Proteins Is Important
My research focuses on MEMO1, a protein closely involved in signal transduction. Studies have shown that MEMO1 plays an important role in cancer spread by relaying signals from Receptor Tyrosine Kinases to other proteins in the cascade. Since then, MEMO1 has been found in various contexts, not only in cancer but in other diseases as well. We aim to identify MEMO1’s global interaction partners to better understand what other pathways or conditions it may influence and, ultimately, how to treat them.
This is at the heart of why studying signal transduction and cellular proteins is so crucial. They drive every cellular function, and by understanding them, we gain deeper insight into how our bodies work and how we can better combat diseases that disrupt these vital processes.

Recent Comments