This summer I am working in Dr. Michael Garabedian’s laboratory at NYU Langone Medical Centre. The focus of my project is to expand upon the groundbreaking research carried out by Dr. Justine Habault. Specifically, my work centers around investigating the mechanism of action of MPC309, a multivalent peptoid conjugate (MPC) that targets castration-resistant and therapy-resistant prostate cancer.
Prostate cancer is a disease that affects the prostate gland in men. It is often treated using medications called Androgen Receptor antagonists, which work by blocking the activity of a specific hormone receptor in the body called the Androgen Receptor. This receptor is involved in the growth and development of prostate cancer cells (Culig & Santer, 2014). However, over time, the cancer cells can develop resistance to these drugs, which means that they are no longer effective in controlling the disease (Levine et al., 2014). This is known as castrate-resistant prostate cancer, which is a highly aggressive form of the disease. This is why finding new treatment approaches that target and modulate the activity of the Androgen Receptor is crucial.
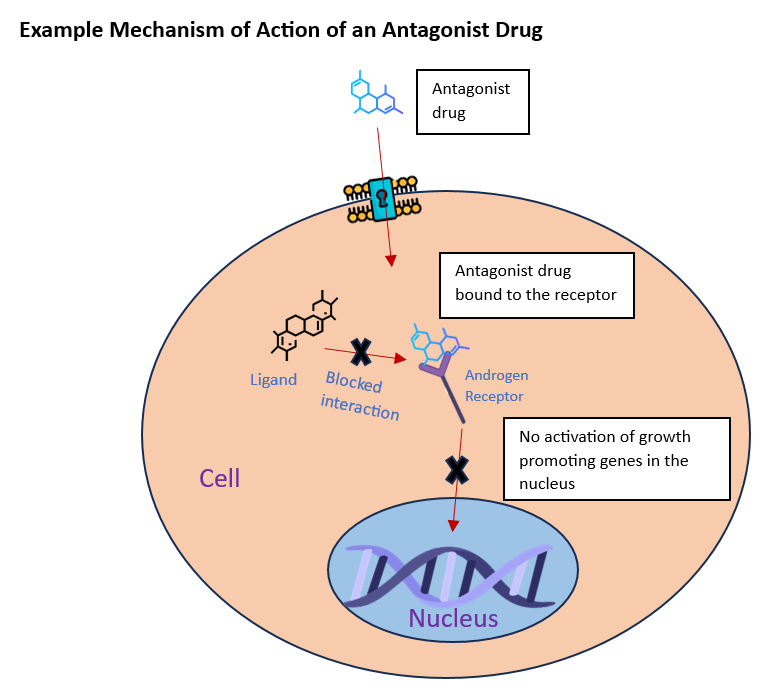
Drugs that are currently on the market usually work by interacting and blocking the activity of one molecule of Androgen Receptor. Now, imagine a drug capable of engaging with multiple targets simultaneously: this would potentially revolutionize the way we approach disease treatment. That is exactly how MPC309, the compound developed by the lab, works. Multivalent peptoid conjugates (MPCs) are molecular constructs capable of engaging with multiple targets simultaneously. By incorporating multiple copies of specific building blocks in their structure, MPCs provide heightened effectiveness and can be tailored to exhibit specific interactions with biological targets, such as proteins or cell surfaces, making them versatile and highly tunable molecular scaffolds (Wang et al., 2016).
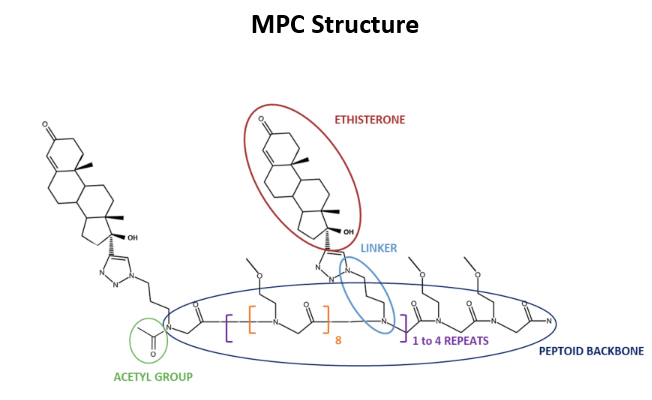
MPC309 consists of a peptoid backbone (a peptide scaffold with chemical modifications that improve its stability), an acetyl group, and three ethisterone groups. Previous research in the lab demonstrated the ability of MPC309 to inhibit castration-resistant prostate cancer cell growth both in vitro (in different cell lines) and in vivo (in mouse models) (Habault et al., 2023). However, how MPC309 exerts its anti-tumoral activity remains to elucidate. That is why my work focused on investigating MPC309 mechanism of action.
First, preliminary data indicated that MPC309 induces the downregulation of genes involved in a mechanism called hypoxia (Habault et al., 2023). Hypoxia is characterized by reduced oxygen levels in the cells/tissues. It plays a crucial role in cancer progression and presents a challenge for conventional cancer therapies as it shields cancer cells from treatment within a protective microenvironment. Therefore, evaluating how the compound affects hypoxia in cancer cells is of significant interest. In this aim, I performed an assay called “IT-hypoxia” that allows the detection of hypoxia in MPC309-treated cells.
In addition to investigating the effect of MPC309 on hypoxia, I looked at the effect of MPC309 on another mechanism called senescence. Cellular senescence is a state where cells in our body stop dividing and enter a dormant phase. It serves as a protective mechanism to prevent damaged or potentially harmful cells from growing uncontrollably. Senescent cells undergo specific changes and stop functioning normally. Treatment with the MPC309 reduces cancer cell proliferation and inhibits tumor growth. Previous studies have shown that the compound does not induce cell death (nor apoptosis or necrosis) but rather cell cycle arrest. Whether the cells are quiescent (temporary state of cellular dormancy) or senescent is still unknown. I performed an assay that measures β-galactosidase (a marker of senescence) in MPC309-treated cells.
In conclusion, MPC309 holds tremendous promise in the field of prostate cancer research and drug development. As previously mentioned, the significance of MPC309 lies in its ability to engage multiple targets simultaneously, providing enhanced affinity, selectivity, and stability. This multivalent nature allows for more effective interactions with cancer cells, potentially leading to improved treatment outcomes and reduced side effects. By exploiting the unique properties of MPC309, we can explore new avenues for targeted drug delivery and personalized medicines.
– Sonia Mecorapaj
Citations:
Culig, Z., & Santer, F. R. (2014). Androgen receptor signaling in prostate cancer. Cancer and Metastasis Reviews, 33(2), 413–427. https://doi.org/10.1007/s10555-013-9474-0
Habault, J., Schneider, J. A., Ha, S., Ruoff, R., Pereira, L., Puccini, J., Ranieri, M., Ayasun, R., Deng, J., Kasper, A. C., Barsagi, D., Wong, K., Zoubeidi, A., Claessens, F., Wise, D. R., Logan, S. K., Kirshenbaum, K. & Garabedian. M. J. (2023). A multivalent peptoid conjugate modulates androgen receptor transcriptional activity to inhibit therapy-resistant prostate cancer. Molecular Cancer Therapeutics (pending publication)
Levine, P. M., Garabedian, M. J., & Kirshenbaum, K. (2014). Targeting the Androgen Receptor with Steroid Conjugates. Journal of Medicinal Chemistry, 57(20), 8224–8237. https://doi.org/10.1021/jm500101h
Wang, Y., Dehigaspitiya, D. C., Levine, P. M., Profit, A. A., Haugbro, M., Imberg-Kazdan, K., Logan, S. K., Kirshenbaum, K., & Garabedian, M. J. (2016). Multivalent Peptoid Conjugates Which Overcome Enzalutamide Resistance in Prostate Cancer Cells. Cancer Research, 76(17), 5124–5132. https://doi.org/10.1158/0008-5472.CAN-16-0385

Recent Comments