I’ve gotten several questions recently from prospective students and their parents asking how undergraduate research works. Who does it? When does it start? What do they do? What is undergraduate research, anyway?
As a professor, I have two jobs. One is to teach students, as I do every day in my classes. The other is to do research, which in my case involves doing experiments, taking data, figuring things out, and then presenting it at conferences and in articles in scientific journals. At big universities like Ohio State and Princeton (where I was before), research is done in groups involving at least one professor plus a small army of post-docs and graduate students. At Richmond, we do the same thing but with undergraduates. We study the same kinds of problems, present at the same national and international conferences, and publish in the same journals. (Does size and lack of graduate students put us at a disadvantage? Only a little bit. Some projects require millions of dollars and dozens of graduate students; other projects don’t. I have to be a little bit careful picking my projects, but there are plenty of unanswered questions out there that I can find the answers to just fine here, thanks. In fact, most of us collaborate with colleagues from other schools, too.)
In our physics department, all six of the tenure-line faculty are research active. (That’s not true at all small colleges, where it’s common for some of the faculty, particularly senior professors who were hired under different expectations, to support their school’s mission in other ways. Some other colleges may also lack the resources to support research at the level we do.) If each of us works with three or four students, the total number of physics students involved in undergraduate research is…well, pretty much all of them, including students only in their first or second year. Note that in our department, research is not just for seniors doing “senior projects” or for only the top honors students. Students typically do research during the year for course credit, and most do research over the summers for money, paid by the University or from a professor’s research grant.
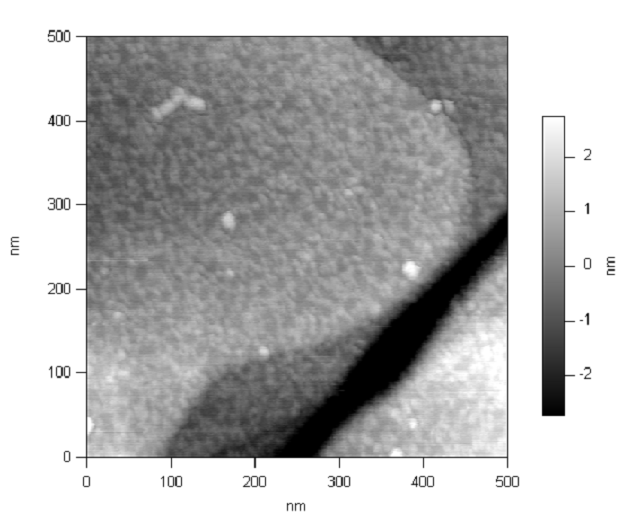
Students here do research on whatever topics the faculty are studying. For me, that means copolymer materials, nanotechnology, and atomic force microscopy. (Other professors here study nuclear physics, particle physics, biological physics, and cosmology.) Although in principle a student could pursue a topic independently, in practice that never happens, simply because figuring out what problems are truly new and interesting (and practical) at the forefront of human knowledge is virtually impossible for a novice. (And if it’s not at the forefront of human knowledge, we’re not generally interested in it here at UR. Topics like “the physics of skiing” are fun and might even be a good learning experience, but that’s just not what we do.) There is no formal process for matching students with faculty; by their sophomore years at the latest, our students generally recognize the benefits of doing research and tend to be drawn to one professor’s lab or another. The professors are always on the lookout for new students too, and in a department as small and informal as ours, connections just happen.
Doing research is a great experience for our students. They are often authors on journal articles, and they get to travel to national and international conferences to present their work–all of which looks pretty good on a resume for graduate school or for jobs. The skills they learn, both specific technical skills and a more general ability to tackle open-ended problems and deal with complexity, are applicable wherever they go. And for me, it’s great to see how students grow from their first tentative steps as incoming students, to confident and capable seniors who go on to do great things. In this way, my two jobs–teaching and research–are really just one job, like two faces of the same coin.