Research Ethics: Standard of Conduct within a given group
- Moral distinction of right & wrong, not necessarily illegal
- As moral norms can vary by culture, typically held to “Western” societal standards
- Manipulation of research data & analysis can have disastrous results
- A.Dzura observation – interesting that hypothetical example referenced a female researcher violating the ethical standards; would need to conduct a detailed review of the text but not sure female was consistently used as hypothetical research?
Key Concepts:
Voluntary Participation & Harmlessness: Participation in research is 100% voluntary, participants may cease participation at any time without consequences and participants will not be harmed as a consequence of research study participation.
- Extensive examples of real life violations of Voluntary Participation & Harmlessness may be found on p.138.
- Informed Consent is document participants acknowledge receiving that explain their rights as a research study participant.
Anonymity & Confidentiality:
- Anonymity in research is when a response/data set cannot be linked to specific participant.
- Assists honest/truthful participation in research study
- Also protects participants from future (eg legal) consequences based on role in research.
- Confidentiality in research is when researcher commits to not divulge participant specific information.
- Leveraged when “anonymity” is not possible.
- Weaker protection for participant as not legally “protected communication”
- In 2002, “Certificate of Confidentiality” introduced by federal government to protect participants where applicable
- Recommendation to deliver confidentiality to participants is for researchers to remove any identifying information once no longer necessary for research study
- Examples of how Confidentiality applies to research studies can be found on p.139
Disclosure: information provided to participants prior to securing their agreement to participate in research study. Transparency is recommended around intent of research, funding, etc. If Disclosure would bias the results of the study, it is recommended that researchers should share with participants in debriefing immediately after participation.
Analysis & Reporting: Expectation is ethical research will communicate with transparency findings of research (favorable & unfavorable), if findings were by design or unexpected outcome, and how data was analyzed to confirm no manipulation to ensure desired or expected outcome of research.
Institutional Review Boards (IRB): Human subject research is governed by federal law. If a research study is using federal money, the study must by overseen and approved by an IRB. Expectation is regardless of funding source, human subject research will follow same standards and procedures as the IBS.
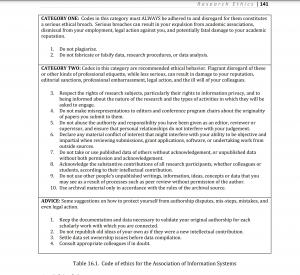
Professional Code of Ethics: Violations assessed at Category 1, more serious and consequences beyond reputational risk, and Category 2. Specific details attached:
http://home.aisnet.org/%20displaycommon.cfm?an=1&subarticlenbr=15
Chapter ends with Ethical Controversy for discussion.
- A.Dzura observation – I believe research was unethical as presented in the textbook as violated modern research ethic concepts below:
- Voluntary Participation – subjects were not aware joining the study despite the public location
- Harmlessness – significant human impact if study results were made public during the study’s contemporary time frame
- Informed consent – none for participants
- Anonymity – none for participants
- Confidentiality – not offered
- Disclosure – not offered

I can’t say that I was particularly surprised by this chapter as it was labelled an “Epilogue”.
It came across as such an after thought. Defining “Ethics is the MORAL (my emphasis) distinction …” was, in my opinion, completely disingenuous.
The chapter emphasized a kind of “professional ethics” approach, and it was almost entirely void of anything even approaching a “moral” discussion. I would try and suggest a moral approach or philosophy the text offered, but it appeared almost entirely amoral.
I do think that it likely represents so much of research in that ethical questions aren’t posed through any sense of moral philosophy, but purely a bureaucratic framework as whether it is following some, seemingly, arbitrary professional association guidelines. I guess by those standards, it follows a “subjective moral” approach, but even at that, I can’t tell what even those are based on.
… Kenny
First off, good job summarizing and secondly, I noted the use of “her/she” in the example early on and actually read it again to see if I missed an example!
I agree with Kenny that it is very narrow in its discussion of ethics and does keep it on a professional side and not on a moral side (or as much). I did think they did a good job showing examples to help with the definitions and seeing some of the differences in each vocabulary. I think some people may think anonymity and confidentiality are the same thing but as the text showed, anonymity is the stronger of the two.
I wonder how strong the tempt or how often it happens that researchers misrepresent or carve out the findings to fit their research or narrative (bias) heading into the study? We all know there is bias in every researcher and after spending so much time on that, it would be tempting for some to skew the results unethically.