Gluten. Glutenaceous, rheological, polymorphic determiner. I’ll back up.
It isn’t wrong to associate gluten and wheat together considering common wheat (Triticum aestivium) contains 85-90% gluten. However, gluten is not one specific protein, nor is it synonymous with the entire wheat kernel. Wheat grain has a highly complex structure and makeup that can be seen below.
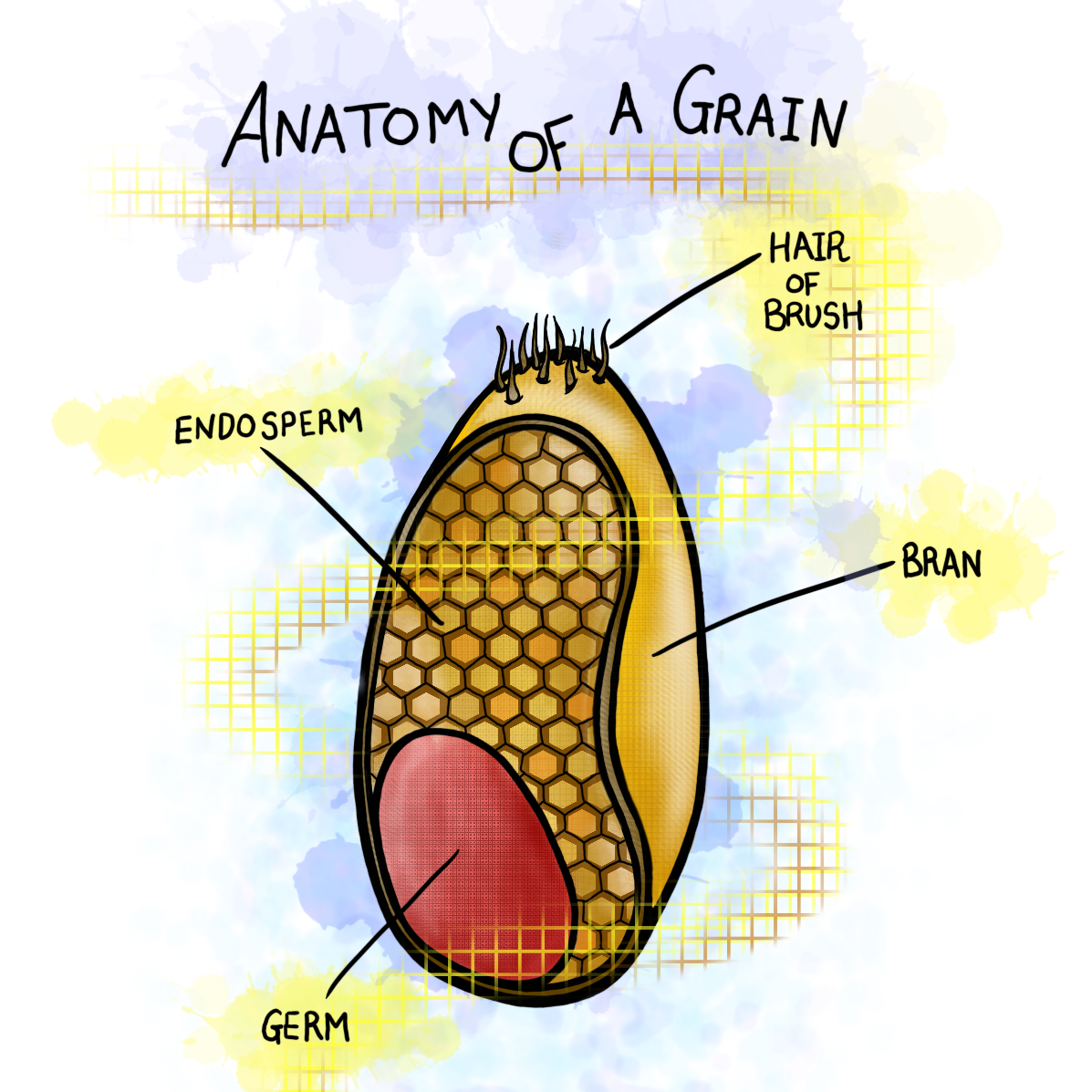
And more simply, here:
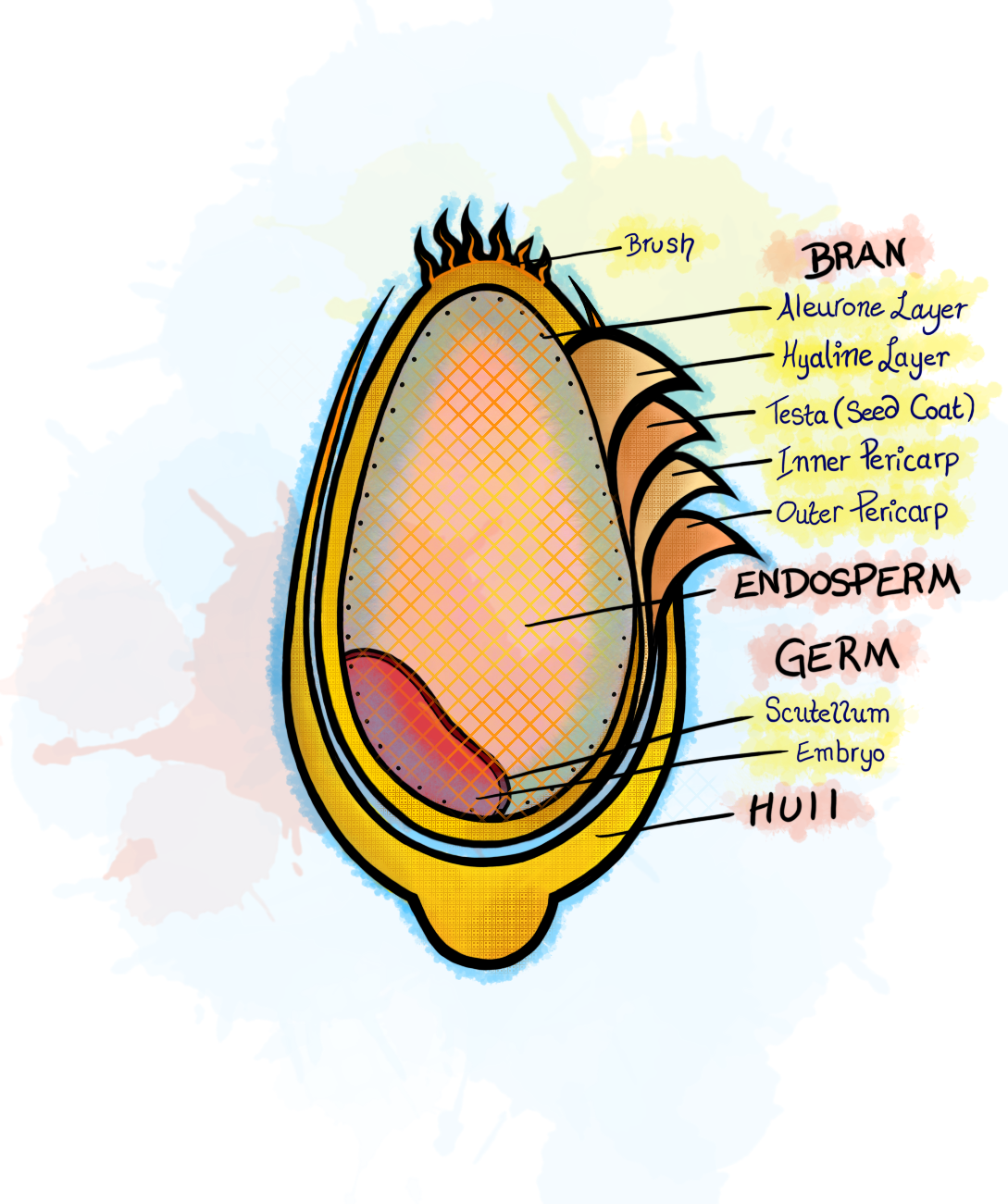
Gluten is the simple name for hundreds of proteins that assist in storing nutrients within wheat. It is found in the endosperm, which I like to think of as the “womb” of wheat since it surrounds the germ (the embryo) and is comprised of protective tissues and proteins.
The bran, which consists of many layers and is broken down extensively in the first illustration, is PACKED with dietary fibers, vitamins, minerals, polyphenols. By definition, when you eat something that is whole wheat or whole grain, you are eating grain that includes the bran and germ.
Without the “whole” designation, the wheat has been stripped of bran and germ and consists of just the endosperm, causing you to miss out on the bran’s nutrients. (Side note: when you are buying bread that is “multi-grain”, it may not actually be “whole grain” since this loophole is often just 10 types of grain that are still conventionally refined to remove the bran and germ).
Given this fact that wheat is so nutrient-rich, why has the amount of gluten-free restaurant visits doubled to 200 million in 4 years? Is bread really that bad for you? These are two questions I hope to answer by the end of this “Cereal,” but first, let’s examine how gluten’s structure interacts with the body.
The two most important proteins to know within the gluten group are named glutenin and gliadin. Think of them as twins.
They are often found in varying ratios throughout wheat and flours because their amounts can determine strength or stretchiness of the protein. This helps when baking, in the case you want a particularly tough or rubber band-like bread. Glutenin and gliadin go hand-in-hand and their ratio is a clue for how the protein might act; whether it be in dough, the plant, or your body.
Since they are twins, I’ll take the soap opera angle here and call gliadin the evil one. When people refer to gluten being harsh on your system or difficult to digest, they aren’t blaming the whole family of gluten proteins. They are blaming the evil twin gliadin.
Gliadin is a prolamin. To advance the evil twin narrative, think of the title of prolamin as the deep-seated part of this protein’s identity that causes all its evil.
Prolamins are proteins that are rich in proline amino acids. Hence the name. If you didn’t take Biochemistry or care about chemistry at all, the important takeaway here is that prolines are highly stable due to a rigid, strong five membered ring. The ring feature of proline is aesthetically pleasing, but when a protein contains multiple, normal processes get tricky.
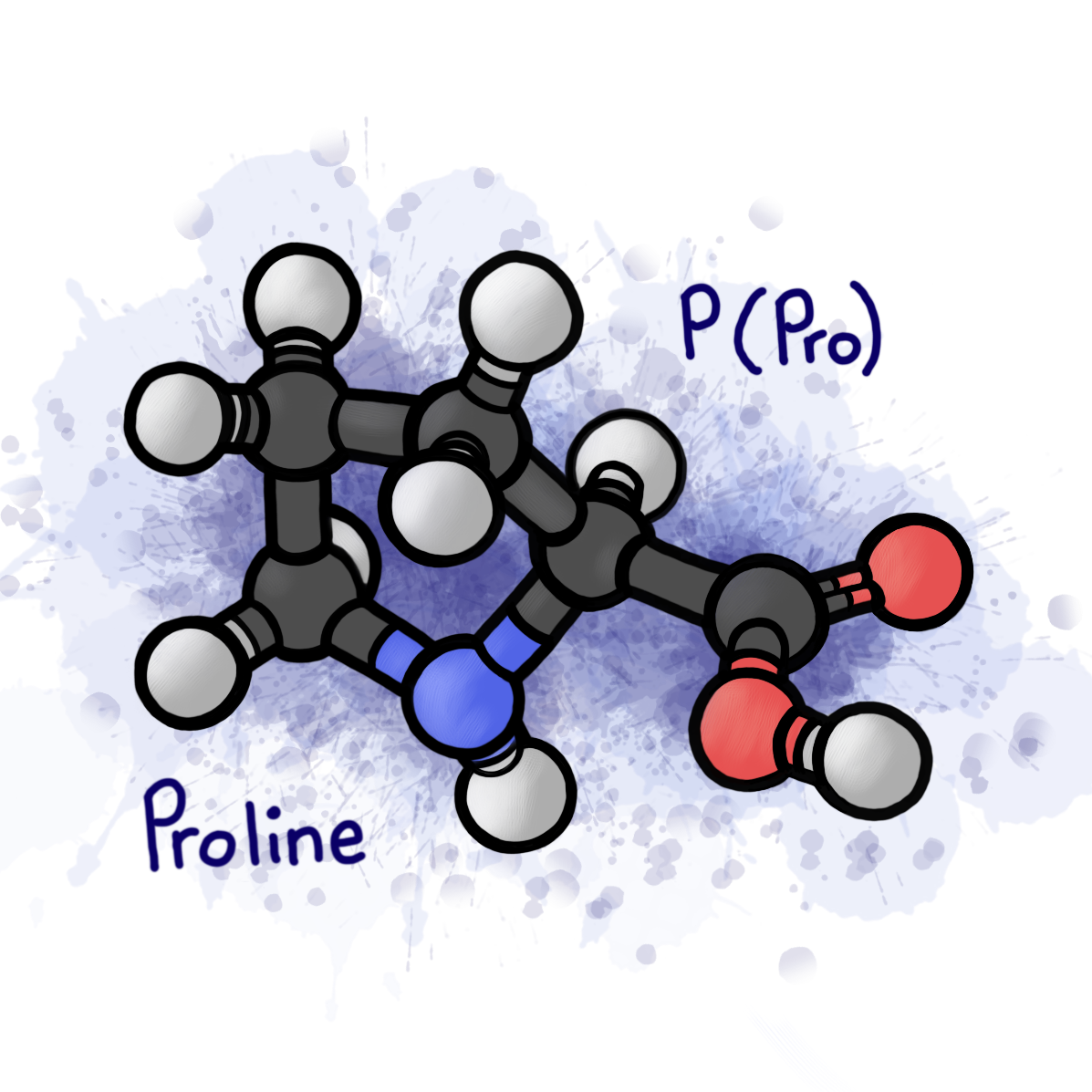
Since gliadins are single-chain polypeptides (long strings of amino acids), they need to be broken down when they enter the body.
The stomach, pancreas, and intestines all have the machinery to deal with these proteins and split them up: enzymes! However, these enzymes do not just go on a free-for-all cutting frenzy. Rather they have very developed, fine motor skills and cut only where they are used to, programmed to, and can cleave perfectly.
For example, pepsin, an enzyme in the stomach that has the first chance to break down proteins, likes to cleave after certain hydrophobic residues. Unfortunately, in the case of gliadin, proline often follows these residues in the chain and, because of proline’s strong ring, it is quite a nuisance and will block pepsin from cutting it.
This sends the unbroken protein of gliadin floating towards the digestive tract until it encounters more enzymes, that, similar to pulling out King Arthur’s sword, would like to try their hand at taking gliadin down.
Chymotrypsin from the pancreas attempts next but runs into a similar problem as pepsin. The proline residues get in the way.
In the intestine, trypsin tries to prove it’s cleaving ability. However, trypsin only likes to cut where arginine and lysine are present, and gliadin does not contain many of those residues. In the end, no enzyme succeeds in cleaving and the full gliadin protein remains the victor.
Gladiator gliadin, if you will.
So what happens to this large protein remaining undigested within the intestine? As you might’ve guessed it is bad news for the digestive tract and especially individuals with celiac disease.
Which is where the story will continue in Part 2…
Art and Images for this article Created by Molly Yon Hin